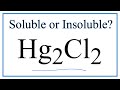
Mercury Compounds and Their Properties
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the common name for Hg2Cl2?
Silver chloride
Mercury two chloride
Mercury one chloride
Lead chloride
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How can Hg2 2+ be best described?
A single mercury ion
A polyatomic ion
A lead ion
A chloride ion
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is generally soluble in water?
Lead chloride
Chlorides in general
Silver chloride
Mercury one chloride
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ion is an exception to the solubility of chlorides?
Calcium ion
Sodium ion
Mercury one ion
Potassium ion
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the solubility status of mercury one chloride in water?
Partially soluble
Highly soluble
Insoluble
Soluble
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge on the mercury ion in mercury two chloride?
Two plus
One plus
Three plus
Zero
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which mercury chloride is soluble in water?
Both are soluble
Both are insoluble
Mercury two chloride
Mercury one chloride
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Solubility of Ionic Compounds
Interactive video
•
9th - 10th Grade

8 questions
Ionic Compounds and Their Properties
Interactive video
•
9th - 10th Grade

10 questions
Chromium Chloride and Aqueous Reactions
Interactive video
•
9th - 10th Grade

6 questions
Net Ionic Equations and Solubility
Interactive video
•
9th - 10th Grade

11 questions
Solubility Rules and Compounds
Interactive video
•
9th - 10th Grade

11 questions
Solubility and Electrolytes Concepts
Interactive video
•
9th - 10th Grade

10 questions
Solubility of Ammonium Chloride
Interactive video
•
9th - 10th Grade

11 questions
Solubility and Chemical Reactions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade