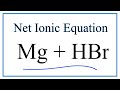
Net Ionic Equations and Reactions
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Sophia Harris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in balancing a net ionic equation?
Identify spectator ions
Split strong electrolytes into ions
Determine the states of substances
Balance the molecular equation
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you balance the bromine atoms in the molecular equation?
Add a coefficient of 2 in front of H2
Add a coefficient of 2 in front of MgBr2
Add a coefficient of 2 in front of Mg
Add a coefficient of 2 in front of HBr
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What state is magnesium in during the reaction?
Liquid
Aqueous
Gas
Solid
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is considered a strong acid in the reaction?
Magnesium bromide
Magnesium
Hydrogen gas
Hydrobromic acid
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of splitting strong electrolytes into ions?
To balance the equation
To determine the states of substances
To identify spectator ions
To calculate the reaction rate
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are spectator ions?
Ions that are present on both sides of the equation
Ions that participate in the reaction
Ions that are only in the reactants
Ions that change state during the reaction
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the net ionic equation for the reaction?
Mg + 2HBr → MgBr2 + H2
2H+ + 2Br- → H2 + Br2
Mg + 2H+ → Mg2+ + H2
Mg2+ + 2Br- → MgBr2
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Understanding Quadratic Functions and Their Forms
Interactive video
•
9th - 10th Grade

11 questions
Understanding Quadratic Functions
Interactive video
•
9th - 10th Grade

6 questions
Understanding Roman Numerals in Ionic Compounds
Interactive video
•
9th - 10th Grade

11 questions
Rocket Propellant Chemistry Quiz
Interactive video
•
9th - 10th Grade

6 questions
Understanding Haploid Number, Ploidy, and Chromosome Replication
Interactive video
•
9th - 10th Grade

11 questions
Understanding the Exact Area of a Yellow Ring
Interactive video
•
9th - 10th Grade

11 questions
Two-Step Equations Quiz
Interactive video
•
9th - 10th Grade

11 questions
Cell Membrane and Homeostasis Quiz
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

15 questions
4:3 Model Multiplication of Decimals by Whole Numbers
Quiz
•
5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
The Best Christmas Pageant Ever Chapters 1 & 2
Quiz
•
4th Grade

12 questions
Unit 4 Review Day
Quiz
•
3rd Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade
Discover more resources for Chemistry

20 questions
Periodic Trends
Quiz
•
10th Grade

29 questions
Physical or Chemical Changes
Quiz
•
9th - 10th Grade

20 questions
Periodic Table & Trends
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
Naming Compounds: Basic Ionic and Covalent Naming
Quiz
•
9th - 12th Grade

20 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade

65 questions
Midterm Review Chem
Quiz
•
9th - 12th Grade