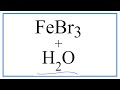
Iron(III) Bromide and Ion Behavior
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the main purpose of examining the solubility of Iron(III) Bromide in water?
To see if it changes color in water
To determine if it can be used as a solid
To find out if it will dissolve and dissociate into ions
To check if it can be used as a gas
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ions are we interested in when applying solubility rules to Iron(III) Bromide?
Iron(II) ion and sulfate ion
Sodium ion and bromide ion
Iron(III) ion and bromide ion
Iron(II) ion and chloride ion
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to solubility rules, how do bromide ions generally behave in water?
They are insoluble with no exceptions
They are soluble with a few exceptions
They are always insoluble
They are soluble only in hot water
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge on the bromide ion?
2+
1-
3+
0
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge on the iron ion in Iron(III) Bromide?
1+
2+
3+
4+
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many bromide ions are present when Iron(III) Bromide dissociates in water?
Three
Two
Four
One
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the term 'aqueous' indicate in the chemical equation for Iron(III) Bromide?
The substance is a solid
The substance is a gas
The substance is dissolved in water
The substance is a liquid
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where is water sometimes shown in the chemical equation for Iron(III) Bromide?
To the left of the reactants
Above the arrow
To the right of the products
Below the arrow
Similar Resources on Wayground

8 questions
Properties and Behavior of NaBr in Water
Interactive video
•
9th - 10th Grade

7 questions
Bromine and Nickel Chemistry Concepts
Interactive video
•
9th - 10th Grade

10 questions
Balancing Chemical Equations and Polyatomic Ions
Interactive video
•
9th - 10th Grade

8 questions
Balancing Chemical Equations Strategies
Interactive video
•
9th - 10th Grade

6 questions
Ammonium Ion and Solubility Concepts
Interactive video
•
9th - 10th Grade

8 questions
Solubility of Barium Bromide
Interactive video
•
9th - 10th Grade

6 questions
Calcium Bromide and Solubility Concepts
Interactive video
•
9th - 10th Grade

9 questions
Iron(III) Bromide Properties and Reactions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade