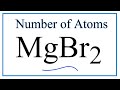
Atoms and Compounds in MgBr2
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Liam Anderson
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What elements are present in the compound MgBr2?
Magnesium and Boron
Magnesium and Bromine
Manganese and Bromine
Manganese and Boron
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many magnesium atoms are present in one formula unit of MgBr2?
None
Two
Three
One
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of atoms in a single unit of MgBr2?
Two
Three
Five
Four
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many bromine atoms are there in one mole of MgBr2?
6.02 x 10^23
1.20 x 10^24
None
3.01 x 10^23
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is Avogadro's number used for in chemistry?
To calculate the mass of an atom
To determine the number of atoms in a mole
To find the volume of a gas
To measure the density of a liquid
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many magnesium atoms are in one mole of MgBr2?
6.02 x 10^23
1.20 x 10^24
3.01 x 10^23
None
Similar Resources on Wayground

11 questions
Methanol Composition and Properties
Interactive video
•
9th - 10th Grade

11 questions
Bromine Reactivity and Redox Reactions
Interactive video
•
9th - 10th Grade

11 questions
Manganese Isotopes and Atomic Mass
Interactive video
•
9th - 10th Grade

11 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

11 questions
Covalent Bonding and Carbon Compounds
Interactive video
•
9th - 10th Grade

8 questions
GCSE Chemistry - What is an Ionic Compound? Ionic Compounds Explained #15
Interactive video
•
9th - 10th Grade

10 questions
Decomposition Reactions of Mercury II Oxide
Interactive video
•
9th - 10th Grade

6 questions
Environmental Impact of CO2 Emissions
Interactive video
•
8th - 12th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

21 questions
Isotopes and Ions
Quiz
•
9th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade