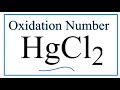
Mercury and Chlorine Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Aiden Montgomery
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the overall charge of the compound HgCl2?
Depends on the environment
Neutral
Negative
Positive
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is mercury not found in the standard oxidation number rules?
It is a halogen
It is a noble gas
It is a non-metal
It is a transition metal
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the typical oxidation number for chlorine in compounds?
-2
-1
0
+1
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many chlorine atoms are present in HgCl2?
4
2
3
1
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What must be the oxidation number of mercury in HgCl2 to balance the compound?
+1
+2
+4
+3
Similar Resources on Wayground

8 questions
Conversion Factors in Chemistry
Interactive video
•
9th - 10th Grade

10 questions
Decomposition Reactions of Mercury II Oxide
Interactive video
•
9th - 10th Grade

7 questions
Cobalt II Chloride and Solubility
Interactive video
•
9th - 10th Grade

7 questions
Conversion of mm of Mercury to Atmospheres
Interactive video
•
9th - 10th Grade

6 questions
Oxidation Numbers and Compounds
Interactive video
•
9th - 10th Grade

6 questions
Balancing Chemical Equations
Interactive video
•
9th - 10th Grade

6 questions
Chlorine and Strontium in Chemistry
Interactive video
•
9th - 10th Grade

7 questions
Oxidation States and Neutral Compounds
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade