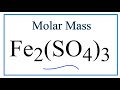
Molar Mass and Chemical Formulas
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Ethan Morris
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for Iron(III) Sulfate?
FeSO4
Fe2(SO4)3
Fe3(SO4)2
Fe2SO4
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many iron atoms are present in the formula Fe2(SO4)3?
2
4
3
1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic mass of sulfur used in the calculation?
40.00 grams per mole
55.85 grams per mole
32.07 grams per mole
16.00 grams per mole
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many oxygen atoms are there in one sulfate ion?
5
4
3
2
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of sulfate ions in Fe2(SO4)3?
2
1
4
3
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final calculated molar mass of Fe2(SO4)3?
399.91 grams per mole
500.00 grams per mole
300.00 grams per mole
250.00 grams per mole
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why might there be slight variations in the calculated molar mass?
Different periodic tables have different atomic masses.
Different chemical formulas.
Incorrect number of atoms.
Calculation errors.
Similar Resources on Wayground

6 questions
World War II Propaganda Quiz
Interactive video
•
9th - 10th Grade

6 questions
Gamma Ray Bursts and Their Impact
Interactive video
•
9th - 10th Grade

6 questions
Fluid Mechanics Quiz
Interactive video
•
9th - 10th Grade

6 questions
Mastering the AI-Driven Hiring Funnel
Interactive video
•
9th - 10th Grade

11 questions
Understanding Boiling Points and Intermolecular Forces
Interactive video
•
9th - 10th Grade

11 questions
Molarity Problems Quiz
Interactive video
•
9th - 10th Grade

11 questions
Understanding Solubility Curves
Interactive video
•
9th - 10th Grade

11 questions
Atomic Mass and Atomic Number Quiz
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Forest Self-Management
Lesson
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

30 questions
Thanksgiving Trivia
Quiz
•
9th - 12th Grade

30 questions
Thanksgiving Trivia
Quiz
•
6th Grade

11 questions
Would You Rather - Thanksgiving
Lesson
•
KG - 12th Grade

48 questions
The Eagle Way
Quiz
•
6th Grade

10 questions
Identifying equations
Quiz
•
KG - University

10 questions
Thanksgiving
Lesson
•
5th - 7th Grade
Discover more resources for Chemistry

88 questions
Test Review
Quiz
•
9th Grade

20 questions
Unit 3, Quiz #6 Practice - Types of Covalent
Quiz
•
9th - 12th Grade

22 questions
Unit 2 Part 1 Rumble
Quiz
•
10th Grade

20 questions
Molar Mass
Quiz
•
9th - 12th Grade

17 questions
Protein Synthesis (Protein Synthesis)
Interactive video
•
9th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade