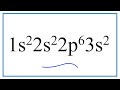
Identifying Elements and Electron Configurations
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Liam Anderson
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the main goal of the video tutorial?
To understand the history of the periodic table
To identify an element based on its electron configuration
To learn about chemical reactions
To explore the properties of metals
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many electrons are present in the given electron configuration?
10
11
12
13
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which element has an atomic number of 12?
Sodium
Silicon
Aluminum
Magnesium
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the number 12 in the context of the video?
It is the number of protons and electrons in the element
It is the number of valence electrons
It is the atomic mass of the element
It is the number of neutrons in the element
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the maximum number of electrons that the s orbital can hold?
4
8
2
6
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which orbital block can hold up to 10 electrons?
s
d
f
p
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the electron configuration that ends in 3s2?
1s2 2s2 2p6 4s2
1s2 2s2 2p6 3d2
1s2 2s2 2p6 3p2
1s2 2s2 2p6 3s2
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Solubility and Chemical Equations
Interactive video
•
9th - 10th Grade

6 questions
Electronegativity and Chemical Bonding
Interactive video
•
9th - 10th Grade

8 questions
Lone Pairs and Molecular Geometry
Interactive video
•
9th - 10th Grade

9 questions
Intermolecular Forces in HF
Interactive video
•
9th - 10th Grade

11 questions
Hexane Molecular Structure and Properties
Interactive video
•
9th - 10th Grade

11 questions
Ionic and Covalent Bonding Concepts
Interactive video
•
9th - 10th Grade

11 questions
Ionic Compounds and Bonding Concepts
Interactive video
•
9th - 10th Grade

11 questions
Ionic Sizes and Electronegativity Trends
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Formative 3BC: Ionic v Covalent Bonds
Quiz
•
9th Grade

10 questions
Exploring Stoichiometry Concepts
Interactive video
•
6th - 10th Grade

20 questions
Mixed Bonding Naming
Quiz
•
9th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Chemical Reactions
Quiz
•
9th Grade

20 questions
Practice: E-Con, Orbital Notation, Noble Gas Notation
Quiz
•
10th Grade

20 questions
Covalent Bonding
Quiz
•
10th Grade