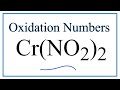
Understanding Chromium and Nitrite Ions
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Liam Anderson
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the absence of a charge sign after chromium in chromium(II) nitrate indicate?
The compound is positively charged.
The compound is neutral.
The compound is negatively charged.
The compound is a gas.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why can chromium have different oxidation numbers?
Because it is a non-metal.
Because it is an alkali metal.
Because it is a transition metal.
Because it is a noble gas.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the ionic charge of the nitrite ion?
-1
+1
+2
0
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do the oxidation numbers of elements in an ion relate to the ion's charge?
They add up to zero.
They are always negative.
They add up to the ion's charge.
They are always positive.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What must the oxidation number of chromium be in chromium(II) nitrate for the compound to be neutral?
+3
+1
+2
+4
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the 'II' in chromium(II) nitrate?
It indicates the number of nitrate ions.
It indicates the charge of the compound.
It indicates the oxidation number of chromium.
It indicates the number of chromium atoms.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where can you find additional help for determining the oxidation number of nitrogen in the nitrite ion?
In the video description.
In the periodic table.
In the appendix.
In the textbook.
Similar Resources on Wayground

7 questions
Nitrogen and Chromium Compounds
Interactive video
•
9th - 10th Grade

8 questions
Chromium(II) Hydroxide Concepts
Interactive video
•
9th - 10th Grade

9 questions
Understanding Chromium Chloride Compounds
Interactive video
•
9th - 10th Grade

10 questions
Oxidation Numbers in K2Cr2O7
Interactive video
•
9th - 10th Grade

6 questions
Atomic Mass and Composition Problems
Interactive video
•
9th - 10th Grade

6 questions
Chromium and Phosphorus Compounds
Interactive video
•
9th - 10th Grade

7 questions
Chromium and Fluoride Compounds
Interactive video
•
9th - 10th Grade

8 questions
Ionic Compounds and Transition Metals
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade