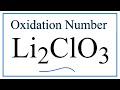
Oxidation Numbers and Chemical Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the overall charge of the compound Li2ClO3?
Positive
Negative
Neutral
Depends on the environment
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which group does lithium belong to on the periodic table?
Group 17
Group 18
Group 1
Group 2
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the typical oxidation number for oxygen in compounds?
-1
-2
+1
+2
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many oxygen atoms are present in Li2ClO3?
4
3
2
1
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the equation used to find the oxidation number of chlorine in Li2ClO3?
x + 3(-2) = 0
2(1) + 3x = 0
2(1) + x + 3(-2) = 0
2x + 3(-2) = 0
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of chlorine in Li2ClO3?
+4
-3
+3
-4
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Who is the presenter of the video?
Dr. A
Dr. B
Dr. D
Dr. C
Similar Resources on Wayground

7 questions
Oxidation Numbers of Hydrogen and Iodine
Interactive video
•
9th - 10th Grade

7 questions
Oxidation Numbers and Compounds
Interactive video
•
9th - 10th Grade

7 questions
Oxidation Numbers and Compound Charges
Interactive video
•
9th - 10th Grade

7 questions
Oxidation Numbers in P2O4
Interactive video
•
9th - 10th Grade

7 questions
Oxidation Numbers in PbO2
Interactive video
•
9th - 10th Grade

7 questions
Oxidation Numbers and Compounds
Interactive video
•
9th - 10th Grade

8 questions
Chlorate Ion and Oxidation States
Interactive video
•
9th - 10th Grade

6 questions
Oxidation Numbers and Periodic Table
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade