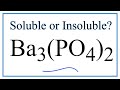
Solubility of Phosphates and Barium
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the solubility status of barium phosphate in water?
Insoluble
Soluble
Partially soluble
Highly soluble
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ion is associated with phosphates in the solubility rules?
PO4 2-
PO3 3-
PO4 3-
PO3 4-
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to solubility rules, which group of elements can form soluble phosphates?
Noble gases
Group 1 elements
Group 2 elements
Transition metals
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of the ammonium ion in phosphate solubility?
It makes phosphates insoluble
It reacts with phosphates to form a gas
It has no effect on solubility
It makes phosphates soluble
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the solubility chart confirm about barium phosphate?
It reacts with water
It is partially soluble in water
It is insoluble in water
It is soluble in water
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where can you find barium on the solubility chart?
In the alkaline earth metals section
In the alkali metals section
In the halogens section
In the transition metals section
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final conclusion about barium phosphate's solubility?
It is insoluble
It is volatile
It is reactive
It is soluble
Similar Resources on Wayground

9 questions
Barium Atom Mass Calculations
Interactive video
•
9th - 10th Grade

9 questions
Understanding Solubility and Chemical Reactions
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Funeral of Gaza kids killed on beach
Interactive video
•
9th - 10th Grade

7 questions
Iron(II) Bicarbonate and Related Concepts
Interactive video
•
9th - 10th Grade

8 questions
Solubility Rules and Hydroxide Salts
Interactive video
•
9th - 10th Grade

8 questions
Binary Ionic Compounds and Their Properties
Interactive video
•
9th - 10th Grade

6 questions
Phytoplankton /Diatoms and Dinoflagellates
Interactive video
•
KG - 9th Grade

9 questions
Phosphate Ions and Vanadium Compounds
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Electron Configuration & Orbital Notation
Quiz
•
9th - 12th Grade