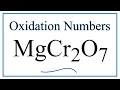
Oxidation States and Chromium Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the overall charge of the compound MgCr2O7?
Positive
Variable
Neutral
Negative
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of magnesium in MgCr2O7?
+1
0
+3
+2
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the oxidation number of chromium initially unknown?
It has multiple oxidation states
It is a non-metal
It is always zero
It is a noble gas
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of oxygen in most compounds?
+1
0
-2
-1
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many chromium atoms are present in MgCr2O7?
4
1
2
3
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of each chromium atom in MgCr2O7?
+6
+3
+4
+5
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total contribution of oxygen atoms to the oxidation state in MgCr2O7?
-8
-10
-12
-14
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of the dichromate ion (Cr2O7)?
1+
2-
1-
2+
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What alternative method can be used to find the oxidation number of chromium?
Using the charge of the dichromate ion
Assuming chromium is a noble gas
Using the periodic table
Guessing based on color
Similar Resources on Wayground

8 questions
Chemical Formulas and Ions
Interactive video
•
9th - 10th Grade

8 questions
Oxidation Numbers and Compound Neutrality
Interactive video
•
9th - 10th Grade

7 questions
Polyatomic Ions and Potassium Chemistry
Interactive video
•
9th - 10th Grade

10 questions
Oxidation States and Numbers in Chemistry
Interactive video
•
9th - 10th Grade

7 questions
Oxidation Numbers and Magnesium Compounds
Interactive video
•
9th - 10th Grade

10 questions
Oxidation States in Lithium Dichromate
Interactive video
•
9th - 10th Grade

9 questions
Silver Dichromate and Its Properties
Interactive video
•
9th - 10th Grade

8 questions
Dichromate and Potassium Ions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
SR&R 2025-2026 Practice Quiz
Quiz
•
6th - 8th Grade

30 questions
Review of Grade Level Rules WJH
Quiz
•
6th - 8th Grade

6 questions
PRIDE in the Hallways and Bathrooms
Lesson
•
12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

15 questions
Subtracting Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

10 questions
Unit 1b Lesson 1 Quick Check
Quiz
•
9th Grade

12 questions
significant figures and calculations
Quiz
•
10th - 12th Grade

20 questions
12.2 Scientific Notation and Significant Figures
Quiz
•
10th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade

12 questions
Atomic Structure and isotopes
Quiz
•
10th Grade