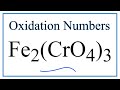
Oxidation Numbers and Iron Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the overall charge of the Iron(III) Chromate compound?
Positive
Neutral
Negative
Variable
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the ionic charge of the chromate ion (CrO4)?
1-
2+
3-
2-
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do the oxidation numbers in a chromate ion add up?
To a positive number
To the charge of the ion
To a negative number
To zero
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of iron in Fe2(CrO4)3?
+4
+2
+6
+3
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many iron atoms are present in Fe2(CrO4)3?
1
2
3
4
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total oxidation number for the iron atoms in Fe2(CrO4)3?
+3
+6
+12
+9
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In oxidation number notation, where is the sign placed?
Before the number
Below the number
After the number
Above the number
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where can you find additional help for finding the oxidation number of chromium in chromate?
In the video description
In the video thumbnail
In the video comments
In the video title
Similar Resources on Wayground

6 questions
Molar Mass and Atomic Mass Concepts
Interactive video
•
9th - 10th Grade

6 questions
Ionic Compounds and Their Properties
Interactive video
•
9th - 10th Grade

7 questions
Potassium Permanganate Properties and Solubility
Interactive video
•
9th - 10th Grade

7 questions
Sodium Chromate and Ionic Charges
Interactive video
•
9th - 10th Grade

9 questions
Lead(II) Chromate Reaction Concepts
Interactive video
•
9th - 10th Grade

8 questions
Oxidation Numbers and Molybdate Ion
Interactive video
•
9th - 10th Grade

11 questions
Iron Ions and Their Properties
Interactive video
•
9th - 10th Grade

11 questions
Chemical Reactions and Acid-Base Theory
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade