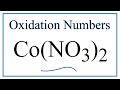
Oxidation Numbers and Cobalt Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Lucas Foster
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the overall charge of cobalt(II) nitrate?
Positive
Negative
Neutral
Variable
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why can't we determine the oxidation state of cobalt just by looking at it?
Because it is a halogen
Because it is a noble gas
Because it is a transition metal
Because it is a non-metal
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of the nitrate ion?
Two plus
One minus
Three minus
Zero
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do oxidation numbers in ions relate to the ion's charge?
They are always positive
They are equal to the number of atoms
They add up to the ion's charge
They are always zero
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of cobalt in cobalt(II) nitrate?
One plus
Two plus
Three plus
Zero
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total oxidation number of the two nitrate ions in cobalt(II) nitrate?
Plus two
Minus one
Minus two
Zero
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where can you find additional help for finding the oxidation number of nitrogen in the nitrate ion?
In the video description
In the textbook
In the glossary
In the appendix
Similar Resources on Wayground

2 questions
Actress Rosalind Russell welcomed by her hometown Waterbury, CT, where she attends movie premiere
Interactive video
•
9th - 10th Grade

2 questions
58th Annual BMI Pop Awards
Interactive video
•
9th - 10th Grade

5 questions
Discolouration
Interactive video
•
KG - 12th Grade

8 questions
The Legislative Branch Of Government - How A Bill Becomes A Law
Interactive video
•
10th - 12th Grade

11 questions
Cellular Respiration and Oxidation Concepts
Interactive video
•
9th - 12th Grade

6 questions
Prince William visits Together as One charity
Interactive video
•
9th - 10th Grade

6 questions
Yohuru Williams - Teaching U.S. History Beyond the Textbook
Interactive video
•
9th - 10th Grade

2 questions
North Country Premiere
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Forest Self-Management
Lesson
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

30 questions
Thanksgiving Trivia
Quiz
•
9th - 12th Grade

30 questions
Thanksgiving Trivia
Quiz
•
6th Grade

11 questions
Would You Rather - Thanksgiving
Lesson
•
KG - 12th Grade

48 questions
The Eagle Way
Quiz
•
6th Grade

10 questions
Identifying equations
Quiz
•
KG - University

10 questions
Thanksgiving
Lesson
•
5th - 7th Grade
Discover more resources for Chemistry

88 questions
Test Review
Quiz
•
9th Grade

20 questions
Unit 3, Quiz #6 Practice - Types of Covalent
Quiz
•
9th - 12th Grade

22 questions
Unit 2 Part 1 Rumble
Quiz
•
10th Grade

20 questions
Molar Mass
Quiz
•
9th - 12th Grade

17 questions
Protein Synthesis (Protein Synthesis)
Interactive video
•
9th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade