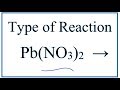
Decomposition Reactions in Chemistry
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Liam Anderson
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of reaction involves a single compound breaking down into simpler substances?
Single replacement reaction
Decomposition reaction
Double replacement reaction
Synthesis reaction
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is a characteristic of a decomposition reaction?
A compound breaks down into two or more products
Two reactants combine to form one product
One element replaces another in a compound
Two compounds exchange ions
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the general form of a decomposition reaction?
AB → A + B
A + BC → B + AC
A + B → AB
AB + CD → AD + CB
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is heat often applied in decomposition reactions?
To combine reactants
To increase the rate of reaction
To break down compounds into simpler substances
To change the state of matter
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the products of the decomposition of lead(II) nitrate?
Lead(II) oxide, nitrogen dioxide, and oxygen
Lead(II) chloride and water
Lead(II) carbonate and carbon dioxide
Lead(II) sulfate and hydrogen
Similar Resources on Wayground

7 questions
Balancing Chemical Equations and Reactions
Interactive video
•
9th - 10th Grade

6 questions
Decomposition Reactions and Products
Interactive video
•
9th - 10th Grade

6 questions
Decomposition Reactions and Copper Compounds
Interactive video
•
9th - 10th Grade

6 questions
Chemical Reactions and Equations
Interactive video
•
9th - 10th Grade

9 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

6 questions
Decomposition Reactions in Chemistry
Interactive video
•
9th - 10th Grade

6 questions
Chemical Reactions and Their Types
Interactive video
•
9th - 10th Grade

8 questions
Decomposition Reactions and Their Characteristics
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Electron Configuration & Orbital Notation
Quiz
•
9th - 12th Grade