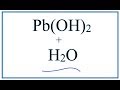
Lead(II) Hydroxide Solubility Concepts
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Mia Campbell
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the main focus of the video regarding lead(II) hydroxide?
Its reaction with acids
Its electrical conductivity
Its color change in water
Its solubility in water
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to check the solubility of a compound first?
To calculate its density
To measure its temperature
To see if it will dissolve or remain solid
To determine its color
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the 'S' stand for in the context of solubility?
Soluble
Solid
Solution
Saturated
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How can a solubility table help in understanding a compound's behavior?
By indicating its solubility status
By listing its chemical formula
By showing its melting point
By providing its molecular weight
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to lead(II) hydroxide when placed in water?
It changes color
It reacts violently
It dissolves completely
It remains mostly solid
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where does the solid lead(II) hydroxide settle in a test tube?
In the middle
It floats
At the top
At the bottom
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the solubility of lead(II) hydroxide in water?
0.155 grams per 100 milliliters
0.0155 grams per 100 milliliters
1.5 grams per 100 milliliters
15 grams per 100 milliliters
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

7 questions
History of Valentine's Day
Interactive video
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

15 questions
Valentine's Day Trivia
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 8 Stoichiometry Review
Quiz
•
10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

19 questions
Stoichiometry, Limiting Reactants, and Percent Yield
Quiz
•
10th Grade

10 questions
Formative 3BD: Ionic Bonds
Quiz
•
9th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

10 questions
Identifying types of reactions
Quiz
•
9th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade