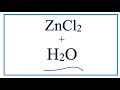
Zinc Chloride and Ionic Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of compound is formed when a metal and a non-metal bond together?
Organic compound
Covalent compound
Metallic compound
Ionic compound
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to check a solubility table when dealing with ionic compounds?
To find the boiling point
To check the electrical conductivity
To confirm the solubility in water
To determine the melting point
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is NOT a characteristic of ionic compounds?
High melting points
Solubility in water
Electrical conductivity in solid state
Formation of ions in solution
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What ions are formed when zinc chloride dissociates in water?
Zinc ion and hydrogen ion
Zinc ion and chloride ion
Chloride ion and oxygen ion
Hydrogen ion and oxygen ion
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of water in the dissociation of zinc chloride?
It acts as a reactant
It acts as a catalyst
It acts as a product
It acts as a solvent
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge on the zinc ion formed during dissociation?
2+
1-
2-
1+
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the result of the reaction between zinc chloride and water?
Precipitation of zinc
Evaporation of water
Dissociation into ions
Formation of a new compound
Create a free account and access millions of resources
Similar Resources on Wayground

9 questions
Solubility and Properties of Sulfides
Interactive video
•
9th - 10th Grade

8 questions
Balancing Chemical Equations Practice
Interactive video
•
9th - 10th Grade

8 questions
Zinc Chloride and Acid-Base Reactions
Interactive video
•
9th - 10th Grade

7 questions
Balancing Chemical Reactions Techniques
Interactive video
•
9th - 10th Grade

8 questions
Zinc Compounds and Naming Conventions
Interactive video
•
9th - 10th Grade

9 questions
Zinc and Iron(III) Chloride Reactions
Interactive video
•
9th - 10th Grade

9 questions
Zinc Chloride Formation and Properties
Interactive video
•
9th - 10th Grade

7 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
SR&R 2025-2026 Practice Quiz
Quiz
•
6th - 8th Grade

30 questions
Review of Grade Level Rules WJH
Quiz
•
6th - 8th Grade

6 questions
PRIDE in the Hallways and Bathrooms
Lesson
•
12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

15 questions
Subtracting Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

10 questions
Unit 1b Lesson 1 Quick Check
Quiz
•
9th Grade

12 questions
significant figures and calculations
Quiz
•
10th - 12th Grade

20 questions
12.2 Scientific Notation and Significant Figures
Quiz
•
10th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade

12 questions
Atomic Structure and isotopes
Quiz
•
10th Grade