Chemical Reactions and Ionic Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Aiden Montgomery
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of compound is formed when a metal and a non-metal combine?
Ionic compound
Covalent compound
Metallic compound
Organic compound
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of a barium ion?
2-
1+
2+
1-
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which group in the periodic table does chlorine belong to?
Group 18
Group 1
Group 2
Group 17
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
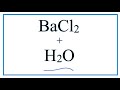
What happens to barium chloride when it is added to water?
It remains unchanged
It reacts to form a gas
It dissolves into ions
It forms a precipitate
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What ions are formed when barium chloride dissolves in water?
Ba2+ and Cl-
Ba+ and Cl-
Ba+ and Cl2-
Ba2- and Cl+
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do we write 'aq' after ions in a chemical equation?
To indicate they are solid
To show they are gaseous
To denote they are dissolved in water
To specify they are in a liquid state
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the term 'aqueous' mean in a chemical equation?
Solid state
Gaseous state
Liquid state
Dissolved in water
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do we ensure a chemical equation is balanced?
By removing some reactants
By adding more reactants
By changing the products
By adjusting coefficients
Similar Resources on Wayground

10 questions
Oxidation Numbers and Barium Dichromate
Interactive video
•
9th - 10th Grade

11 questions
Precipitation Reactions and Ion Testing
Interactive video
•
9th - 10th Grade

9 questions
Barium Compounds and Reactions
Interactive video
•
9th - 10th Grade

6 questions
Production and Legal Aspects Overview
Interactive video
•
9th - 10th Grade

6 questions
Barium Selenide and Ionic Compounds
Interactive video
•
9th - 10th Grade

7 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

9 questions
Barium and Bromine Compounds
Interactive video
•
9th - 10th Grade

10 questions
Ionic Compounds and Their Properties
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Electron Configuration & Orbital Notation
Quiz
•
9th - 12th Grade