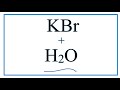
Dissociation and Properties of KBr
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of compound is potassium bromide (KBr)?
Molecular compound
Metallic compound
Ionic compound
Covalent compound
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What charge does potassium (K) have in the periodic table?
+2
-1
+1
0
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In which group of the periodic table is bromine (Br) found?
Group 2
Group 1
Group 17
Group 18
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to KBr when it is dissolved in water?
It forms a precipitate
It dissociates into ions
It remains unchanged
It evaporates
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the ions formed when KBr dissociates in water?
K2+ and Br2-
K2+ and Br-
K- and Br+
K+ and Br-
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does 'aq' signify when written after an ion?
The ion is in solid form
The ion is in gaseous form
The ion is dissolved in water
The ion is in liquid form
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it unnecessary to write H2O on the product side of the equation?
Because water evaporates
Because 'aq' indicates the ions are dissolved in water
Because the reaction does not occur
Because water is not involved in the reaction
Similar Resources on Wayground

8 questions
Potassium Hypochlorite and Ionic Charges
Interactive video
•
9th - 10th Grade

8 questions
Potassium Bromide Properties and Formulas
Interactive video
•
9th - 10th Grade

7 questions
Chemical Properties of Potassium Nitrate
Interactive video
•
9th - 10th Grade

11 questions
Net Ionic Equations and Spectator Ions
Interactive video
•
9th - 10th Grade

8 questions
Ionic Compounds and Chemical Reactions
Interactive video
•
9th - 10th Grade

11 questions
Net Ionic Equations and Spectator Ions
Interactive video
•
9th - 10th Grade

6 questions
Naming Acids with Oxygen Ions Quiz
Interactive video
•
9th - 10th Grade

7 questions
Chlorate Ion and Potassium Compounds
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade