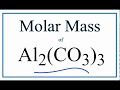
Molar Mass and Chemical Composition
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Emma Peterson
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic mass of aluminum as found on the periodic table?
16.00
12.01
26.98
53.96
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many oxygen atoms are present in the carbonate group of aluminum carbonate?
2
1
4
3
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total mass of the carbonate group (CO3) in aluminum carbonate?
180.03
233.99
60.01
48.00
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final molar mass of aluminum carbonate?
26.98 g/mol
180.03 g/mol
53.96 g/mol
233.99 g/mol
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to use units when calculating molar mass?
To ensure accuracy
To make calculations easier
To avoid confusion with other measurements
To impress others
Similar Resources on Wayground

2 questions
CLEAN : Thousands compete in the Great Ethiopian Run in Addis Ababa
Interactive video
•
9th - 10th Grade

2 questions
Marble beats Stammers in Wightman Cup match; Budge beats Riggs in Casino Invitational
Interactive video
•
9th - 10th Grade

6 questions
Reporter at scene of Nice knife attack
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Israel Palestinians: more clashes in Hebron as unrest worses
Interactive video
•
9th - 10th Grade

2 questions
Stars /UY Scuti Stars/Largest Star in the Milky Way Galaxy
Interactive video
•
KG - 9th Grade

2 questions
VOICED : Oposicion venezolana lista para megamarcha por el revocatorio
Interactive video
•
9th - 12th Grade

6 questions
CLEAN : 'There will be no New Year's Eve party' : third Austrian lockdown to start on December 26
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade