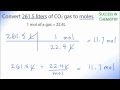
Unit Conversions and Factor Label Method
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Medium
Lucas Foster
Used 1+ times
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the initial volume of CO2 gas that needs to be converted to moles?
100 liters
11.7 liters
22.4 liters
261.5 liters
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the factor label method, what unit is placed on the bottom to cancel out liters?
Moles
Liters
Grams
Kilograms
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the conversion factor used to convert liters of gas to moles?
1 liter = 22.4 moles
1 mole = 22.4 liters
1 mole = 1 liter
1 liter = 1 mole
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
After performing the calculation, how many moles of CO2 gas are obtained?
22.4 moles
11.7 moles
261.5 moles
0.5 moles
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the alternative method mentioned for converting liters to moles?
Subtracting 22.4
Adding 22.4
Dividing by 22.4
Multiplying by 22.4
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the factor label method preferred for unit conversions?
It can be used for any unit conversion
It requires less calculation
It is more accurate
It is faster
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final result of the conversion from liters to moles?
0.5 moles
22.4 moles
11.7 moles
261.5 moles
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Rigging Terminology and Concepts
Interactive video
•
9th - 12th Grade

11 questions
Graphing Quadratics in Vertex Form
Interactive video
•
9th - 12th Grade

11 questions
Understanding Car Dealership Tactics
Interactive video
•
9th - 12th Grade

11 questions
Understanding Real World Advice and Business Needs
Interactive video
•
9th - 12th Grade

11 questions
Factoring Quadratics: Day 2A Insights
Interactive video
•
8th - 12th Grade

6 questions
Scientific Method - TAT Project
Interactive video
•
10th Grade

11 questions
Understanding Quadratic Graphs Concepts
Interactive video
•
8th - 10th Grade

11 questions
Understanding Polynomial Zeros and Multiplicity
Interactive video
•
8th - 10th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Energy Transformations
Quiz
•
9th - 12th Grade

20 questions
Periodic Table & Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

24 questions
Identifying Types of Chemical Reactions
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

10 questions
Identifying Types of Chemical Reactions
Interactive video
•
6th - 10th Grade