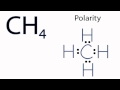
Understanding CH4 Molecular Structure and Polarity
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Ethan Morris
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the initial step in determining the polarity of CH4?
Analyzing its color
Examining the Lewis structure
Measuring its temperature
Checking its solubility
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to visualize CH4 in three dimensions?
To understand the spread of hydrogen atoms
To measure its weight
To determine its color
To calculate its density
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What shape does the CH4 molecule form?
Square planar
Linear
Trigonal planar
Tetrahedral
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How are the hydrogen atoms arranged around the carbon atom in CH4?
Randomly
In a circular pattern
Equidistantly
In a straight line
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the net polarity of the CH4 molecule?
Metallic
Polar
Nonpolar
Ionic
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does CH4 have no net difference in polarity?
Because of its color
Due to its symmetrical shape
Because it is a gas
Due to its high temperature
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the symmetrical arrangement of hydrogen atoms in CH4?
It causes CH4 to be reactive
It makes CH4 a polar molecule
It makes CH4 a solid at room temperature
It results in no areas of charge imbalance
Similar Resources on Wayground

11 questions
Ethanol Molecular Properties and Interactions
Interactive video
•
9th - 10th Grade

8 questions
Molecular Dipoles and Electronegativity
Interactive video
•
9th - 12th Grade

11 questions
The Fascinating Properties of Water and Its Role in Life
Interactive video
•
9th - 10th Grade

7 questions
Oxidation Numbers and Methane
Interactive video
•
9th - 10th Grade

11 questions
Understanding SO2: Structure and Polarity
Interactive video
•
9th - 10th Grade

11 questions
Intermolecular Forces and Molecular Structure
Interactive video
•
9th - 10th Grade

6 questions
Molecular Polarity Quiz
Interactive video
•
9th - 10th Grade

11 questions
Hydrophilic and Hydrophobic Molecules
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade