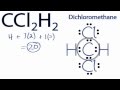
Valence Electrons and Lewis Structures
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Ethan Morris
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does carbon have in the CCl2H2 Lewis structure?
8
2
6
4
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which element is placed at the center of the CCl2H2 Lewis structure?
Oxygen
Carbon
Hydrogen
Chlorine
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why are hydrogens placed on the outside of the Lewis structure?
They have the most valence electrons
They are the least electronegative
They only need two valence electrons
They are heavier than other atoms
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to form chemical bonds in the CCl2H2 structure?
12
16
4
8
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons available for the CCl2H2 Lewis structure?
18
16
20
22
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons do the chlorines have in their outer shells?
6
7
8
9
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of fulfilling the octets in the Lewis structure?
It ensures atoms have full outer shells
It reduces the number of valence electrons
It increases the electronegativity
It changes the atomic mass
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final step in completing the CCl2H2 Lewis structure?
Adding more hydrogen atoms
Ensuring all atoms have full outer shells
Removing excess electrons
Changing the central atom
Similar Resources on Wayground

6 questions
Cl2O Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

6 questions
Iodine and Chlorine Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
Arsenic and Fluorine Bonding Concepts
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in OCl2
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in HOCl Molecule
Interactive video
•
9th - 10th Grade

6 questions
BrO- Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in S2Cl2 Structure
Interactive video
•
9th - 10th Grade

7 questions
NO2 Structure and Valence Electrons
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
SR&R 2025-2026 Practice Quiz
Quiz
•
6th - 8th Grade

30 questions
Review of Grade Level Rules WJH
Quiz
•
6th - 8th Grade

6 questions
PRIDE in the Hallways and Bathrooms
Lesson
•
12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

15 questions
Subtracting Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

10 questions
Unit 1b Lesson 1 Quick Check
Quiz
•
9th Grade

12 questions
significant figures and calculations
Quiz
•
10th - 12th Grade

20 questions
12.2 Scientific Notation and Significant Figures
Quiz
•
10th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade

12 questions
Atomic Structure and isotopes
Quiz
•
10th Grade