Formal Charges and Lewis Structures
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Emma Peterson
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
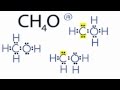
How many valence electrons are present in the CH4O Lewis structure?
18
16
14
12
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it unusual to see lone pairs on Carbon in Lewis structures?
Carbon is a noble gas.
Carbon prefers to have a positive charge.
Carbon usually forms four bonds.
Carbon is less electronegative than Oxygen.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of calculating formal charges in Lewis structures?
To calculate the molecular weight.
To identify the molecular geometry.
To find the most stable structure.
To determine the number of valence electrons.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the top structure, what are the formal charges on Carbon and Oxygen?
Carbon: +1, Oxygen: -1
Carbon: 0, Oxygen: 0
Carbon: +2, Oxygen: -1
Carbon: -1, Oxygen: +2
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which structure has formal charges closest to zero?
The structure with negative charge on Carbon.
The structure with positive charge on Oxygen.
The structure with negative charge on Oxygen and positive on Carbon.
The structure with no charges on any atoms.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important for formal charges to be close to zero?
It changes the molecular geometry.
It affects the color of the compound.
It increases the molecular weight.
It indicates a more stable structure.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the most appropriate Lewis structure for CH4O based on the video?
The structure with lone pairs on Carbon.
The structure with a negative charge on Carbon.
The structure with a positive charge on Oxygen.
The structure with formal charges closest to zero.
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade