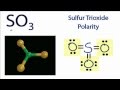
SO3 Molecular Structure and Polarity
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Aiden Montgomery
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bond angle in the trigonal planar geometry of SO3?
180 degrees
120 degrees
109.5 degrees
90 degrees
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is SO3 considered nonpolar based on its Lewis structure?
It has a linear shape.
The oxygens are asymmetrical.
The oxygens are symmetrical and spread out evenly.
It has a tetrahedral shape.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the shape of the SO3 molecule in three dimensions?
Linear
Bent
Trigonal planar
Tetrahedral
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does the 3D shape of SO3 contribute to its nonpolar nature?
It makes the molecule linear.
It creates a positive pole.
It ensures no poles are formed.
It creates a negative pole.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the electrostatic potential of SO3 indicate about its polarity?
It has a positive side.
It has a negative side.
There are no distinct poles.
It is highly polar.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What conclusion can be drawn about the polarity of SO3 from its electronic structure?
SO3 is nonpolar because it is linear.
SO3 is nonpolar due to the absence of poles.
SO3 is polar because it has a positive and negative side.
SO3 is polar due to its asymmetrical shape.
Similar Resources on Wayground

8 questions
Polarity and Properties of CHCl3
Interactive video
•
9th - 10th Grade

7 questions
Properties and Behavior of C3H8
Interactive video
•
9th - 10th Grade

10 questions
Properties and Structure of SF6
Interactive video
•
9th - 10th Grade

10 questions
Polarity and Bonding in AlBr3
Interactive video
•
9th - 10th Grade

8 questions
Intermolecular Forces in HBr
Interactive video
•
9th - 10th Grade

7 questions
Bond Types and Electronegativity Concepts
Interactive video
•
9th - 10th Grade

6 questions
Polarity and Structure of I2 Molecule
Interactive video
•
9th - 10th Grade

7 questions
Polarity and Structure of SOCl2
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade