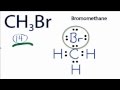
Valence Electrons in CH3Br
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Aiden Montgomery
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many total valence electrons are present in CH3Br?
12
14
16
18
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is placed at the center of the CH3Br Lewis structure?
Hydrogen
Oxygen
Bromine
Carbon
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to form chemical bonds between the atoms in CH3Br?
6
10
8
12
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many electrons does each hydrogen atom have in the CH3Br structure?
4
1
2
3
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of electrons around the Bromine atom in CH3Br?
6
7
8
9
Similar Resources on Wayground

6 questions
Understanding Elements in the Periodic Table
Interactive video
•
9th - 10th Grade

7 questions
thành phần cấu tạo nguyên tử
Interactive video
•
10th Grade

11 questions
The Science and Imagination of Human Flight
Interactive video
•
9th - 10th Grade

11 questions
Understanding Atoms and Subatomic Particles
Interactive video
•
9th - 10th Grade

11 questions
Cell Membrane and Homeostasis Quiz
Interactive video
•
9th - 10th Grade

11 questions
Understanding Molecular and Empirical Formulas
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 4/5-Covalent Bonding/Nomenclature
Quiz
•
10th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
Ions
Quiz
•
10th Grade

25 questions
VSPER Shape Quiz
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

61 questions
KAP Chemistry Covalent Test Review
Quiz
•
10th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade