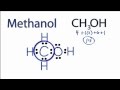
Valence Electrons in Methanol
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does carbon have in the methanol molecule?
4
6
8
2
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in methanol?
10
12
14
16
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is placed at the center of the methanol molecule?
Carbon
Oxygen
Nitrogen
Hydrogen
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to form chemical bonds in methanol?
14
12
10
8
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What rule is satisfied when all atoms in methanol have full valence shells?
Quadruple rule
Duet rule
Octet rule
Triple rule
Similar Resources on Wayground

7 questions
Valence Electrons in N2O4 Structure
Interactive video
•
9th - 10th Grade

6 questions
Polarity of O2 Molecule Concepts
Interactive video
•
9th - 10th Grade

8 questions
Valence Electrons in C2Cl6
Interactive video
•
9th - 10th Grade

8 questions
Valence Electrons in CHF3
Interactive video
•
9th - 10th Grade

8 questions
CCl2O Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

6 questions
NO2Cl Structure and Bonding Concepts
Interactive video
•
9th - 10th Grade

7 questions
Bromine Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

7 questions
Lewis Structures and Valence Electrons
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade