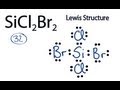
Lewis Structures and Valence Electrons
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Sophia Harris
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in the Si2Br2 molecule?
32
24
40
16
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is silicon placed in the center of the Si2Br2 Lewis structure?
It has the highest electronegativity.
It has the lowest electronegativity.
It has the most valence electrons.
It is the largest atom.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many electrons are placed between atoms to form chemical bonds in Si2Br2?
8
6
4
2
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of filling the octets in the Lewis structure?
To change the molecule's shape
To reduce the number of electrons
To stabilize the molecule
To increase the molecule's size
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What confirms that the Lewis structure for Si2Br2 is complete?
All atoms have 12 valence electrons.
All atoms have 10 valence electrons.
All atoms have 6 valence electrons.
All atoms have 8 valence electrons.
Similar Resources on Wayground

9 questions
Understanding Compromises in the Constitution
Interactive video
•
10th - 12th Grade

9 questions
Intro Photosynthesis
Interactive video
•
10th Grade

6 questions
The Fall of Rome
Interactive video
•
8th Grade

6 questions
Galaxies Part 1
Interactive video
•
8th Grade

8 questions
Understanding Sole Proprietorships
Interactive video
•
9th - 12th Grade

6 questions
Empirical Formulas, Molecular Formulas, and Molecular Geometry
Interactive video
•
10th Grade
Popular Resources on Wayground

10 questions
Forest Self-Management
Lesson
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

30 questions
Thanksgiving Trivia
Quiz
•
9th - 12th Grade

30 questions
Thanksgiving Trivia
Quiz
•
6th Grade

11 questions
Would You Rather - Thanksgiving
Lesson
•
KG - 12th Grade

48 questions
The Eagle Way
Quiz
•
6th Grade

10 questions
Identifying equations
Quiz
•
KG - University

10 questions
Thanksgiving
Lesson
•
5th - 7th Grade
Discover more resources for Chemistry

88 questions
Test Review
Quiz
•
9th Grade

20 questions
Unit 3, Quiz #6 Practice - Types of Covalent
Quiz
•
9th - 12th Grade

22 questions
Unit 2 Part 1 Rumble
Quiz
•
10th Grade

20 questions
Molar Mass
Quiz
•
9th - 12th Grade

17 questions
Protein Synthesis (Protein Synthesis)
Interactive video
•
9th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade