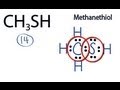
Valence Electrons in CH3SH
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Sophia Harris
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in CH3SH?
16
14
18
12
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is placed at the center of the CH3SH Lewis structure?
Oxygen
Carbon
Sulfur
Hydrogen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to form chemical bonds in CH3SH before completing the octet?
10
8
12
6
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the minimum number of electrons needed for hydrogen to be stable in a Lewis structure?
1
2
4
8
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does sulfur have in the completed Lewis structure of CH3SH?
10
8
6
12
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons around carbon in the CH3SH Lewis structure?
6
8
10
12
Similar Resources on Wayground

9 questions
Intro Photosynthesis
Interactive video
•
10th Grade

6 questions
The Fall of Rome
Interactive video
•
8th Grade

6 questions
Galaxies Part 1
Interactive video
•
8th Grade

8 questions
Understanding Sole Proprietorships
Interactive video
•
9th - 12th Grade

9 questions
Understanding Compromises in the Constitution
Interactive video
•
10th - 12th Grade

6 questions
Empirical Formulas, Molecular Formulas, and Molecular Geometry
Interactive video
•
10th Grade
Popular Resources on Wayground

10 questions
Forest Self-Management
Lesson
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

30 questions
Thanksgiving Trivia
Quiz
•
9th - 12th Grade

30 questions
Thanksgiving Trivia
Quiz
•
6th Grade

11 questions
Would You Rather - Thanksgiving
Lesson
•
KG - 12th Grade

48 questions
The Eagle Way
Quiz
•
6th Grade

10 questions
Identifying equations
Quiz
•
KG - University

10 questions
Thanksgiving
Lesson
•
5th - 7th Grade
Discover more resources for Chemistry

88 questions
Test Review
Quiz
•
9th Grade

20 questions
Unit 3, Quiz #6 Practice - Types of Covalent
Quiz
•
9th - 12th Grade

22 questions
Unit 2 Part 1 Rumble
Quiz
•
10th Grade

20 questions
Molar Mass
Quiz
•
9th - 12th Grade

17 questions
Protein Synthesis (Protein Synthesis)
Interactive video
•
9th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade