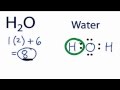
Valence Electrons and Water Structure
Interactive Video
•
Chemistry
•
6th - 8th Grade
•
Hard
Liam Anderson
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does a single hydrogen atom have?
1
2
6
8
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in a water molecule?
6
8
12
10
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where is the oxygen atom placed in the Lewis structure of water?
Next to one hydrogen
In the center
Above the hydrogens
On the outside
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does each hydrogen atom need to have a full outer shell?
1
2
4
8
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What rule is satisfied when the oxygen atom in water has eight valence electrons?
Octet rule
Triple bond rule
Duet rule
Valence rule
Similar Resources on Wayground

11 questions
Valence Electrons and Shells in Atoms Using the Periodic Table
Interactive video
•
6th - 8th Grade

11 questions
Valence Electrons and Lewis Dot Diagrams
Interactive video
•
6th - 8th Grade

11 questions
Valence Electrons and Atomic Structure
Interactive video
•
6th - 8th Grade

11 questions
Exploring Lewis Dots in Chemistry
Interactive video
•
6th - 10th Grade

11 questions
Drawing Lewis Structures for Ionic Compounds
Interactive video
•
6th - 10th Grade

6 questions
NO2Cl Structure and Bonding Concepts
Interactive video
•
9th - 10th Grade

7 questions
Bromine Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

7 questions
Lewis Structures and Valence Electrons
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Physical and Chemical Properties
Quiz
•
8th Grade

20 questions
States of Matter
Quiz
•
8th Grade

20 questions
Counting Atoms Practice
Quiz
•
8th Grade

20 questions
Solutes, Solvents, Solutions
Quiz
•
6th - 8th Grade

18 questions
Law of Conservation of Mass
Lesson
•
8th Grade

20 questions
Chemical and Physical Properties and Changes
Quiz
•
7th Grade

22 questions
SCIENCE LAB EQUIPMENT
Quiz
•
5th - 12th Grade

15 questions
Acids and Bases Review
Quiz
•
8th Grade