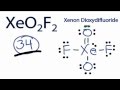
XeO2F2 Structure and Valence Electrons
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Liam Anderson
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in XeO2F2?
32
34
36
38
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is placed at the center in the initial structure of XeO2F2?
Xenon
Fluorine
Hydrogen
Oxygen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used after forming the initial chemical bonds in XeO2F2?
4
6
8
10
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of forming double bonds with oxygen in the revised structure?
To increase the number of valence electrons
To eliminate the +2 formal charge on Xenon
To decrease the molecular weight
To change the molecular shape
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formal charge on each atom in the final structure of XeO2F2?
+2
-1
+1
0
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used in the final structure of XeO2F2?
32
34
38
36
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What makes the final structure of XeO2F2 the best structure?
All formal charges are zero
It uses fewer valence electrons
It is the most complex structure
It has a unique shape
Similar Resources on Wayground
Popular Resources on Wayground

20 questions
Halloween Trivia
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Order of Operations
Quiz
•
5th Grade

20 questions
Halloween
Quiz
•
5th Grade

16 questions
Halloween
Quiz
•
3rd Grade

12 questions
It's The Great Pumpkin Charlie Brown
Quiz
•
1st - 5th Grade

20 questions
Possessive Nouns
Quiz
•
5th Grade

10 questions
Halloween Traditions and Origins
Interactive video
•
5th - 10th Grade
Discover more resources for Chemistry

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade

20 questions
2.6 Electron Configurations and Orbital Notations
Quiz
•
10th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

16 questions
Naming Ionic Compounds
Quiz
•
9th - 11th Grade

10 questions
Isotopes
Quiz
•
9th - 12th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

15 questions
Intro to Atoms
Quiz
•
8th - 10th Grade