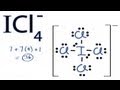
ICl4- Structure and Valence Electrons
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Liam Anderson
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does iodine have in the ICl4- structure?
7
6
8
5
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons used in the ICl4- structure?
32
34
36
38
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is placed at the center of the ICl4- structure?
Chlorine
Iodine
Hydrogen
Oxygen
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are initially used to form bonds between iodine and chlorine?
4
8
6
10
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why can iodine hold more than eight valence electrons in the ICl4- structure?
It is in period 3 of the periodic table.
It is a noble gas.
It has a high electronegativity.
It is in period 5 of the periodic table.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formal charge on the iodine atom in the ICl4- structure?
+1
-2
-1
0
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why are brackets used around the ICl4- Lewis structure?
To indicate it is a molecule.
To show it is an ion with a negative charge.
To separate it from other structures.
To highlight the central atom.
Popular Resources on Wayground

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
4:3 Model Multiplication of Decimals by Whole Numbers
Quiz
•
5th Grade

10 questions
The Best Christmas Pageant Ever Chapters 1 & 2
Quiz
•
4th Grade

12 questions
Unit 4 Review Day
Quiz
•
3rd Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

15 questions
Solving Equations with Variables on Both Sides Review
Quiz
•
8th Grade
Discover more resources for Chemistry

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Unit 3, Quiz #6 Practice - Types of Covalent
Quiz
•
9th - 12th Grade

17 questions
Protein Synthesis (Protein Synthesis)
Interactive video
•
9th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Exploring Ionic and Covalent Bonding Concepts
Interactive video
•
6th - 10th Grade

148 questions
Fall TEKS Review Chemistry
Quiz
•
9th - 12th Grade

20 questions
Unit 5 - Chemical Reactions Refresh
Quiz
•
9th - 12th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade