Lewis Structures and Valence Electrons
Interactive Video
•
Chemistry
•
10th - 12th Grade
•
Hard
Mia Campbell
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
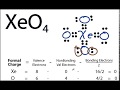
How many valence electrons does Xenon have in the XeO4 molecule?
12
6
8
10
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in XeO4?
24
28
32
36
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the initial step in drawing the Lewis structure for XeO4?
Draw double bonds
Calculate formal charges
Place Xenon in the center
Place Oxygen in the center
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to check formal charges in a Lewis structure?
To verify the octet rule
To confirm the structure's stability
To ensure the structure is colorful
To count the number of atoms
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What formal charge does Xenon initially have in the first Lewis structure attempt?
-1
0
+2
+4
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What adjustment is made to the Lewis structure to improve formal charges?
Add more electrons
Introduce double bonds
Remove an oxygen atom
Change the central atom
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many double bonds are introduced in the revised Lewis structure of XeO4?
1
2
3
4
Create a free account and access millions of resources
Similar Resources on Wayground

7 questions
Krypton and Valence Electrons Concepts
Interactive video
•
10th - 12th Grade

11 questions
Molecular Structures and Bonding Concepts
Interactive video
•
10th - 12th Grade

11 questions
Formal Charge and Lewis Structures
Interactive video
•
10th - 12th Grade

11 questions
Formal Charges and Lewis Structures
Interactive video
•
9th - 12th Grade

11 questions
Chlorine and HClO4 Structure Concepts
Interactive video
•
10th - 12th Grade

11 questions
Lewis Structures and Valence Electrons
Interactive video
•
10th - 12th Grade

9 questions
Understanding H2SO3 and Lewis Structures
Interactive video
•
10th - 12th Grade

10 questions
Lewis Structures and Resonance Concepts
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade