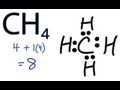
Understanding Methane and Valence Electrons
Interactive Video
•
Chemistry
•
6th - 8th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for methane?
CH4
CH3
C2H6
C2H4
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does carbon have?
6
4
8
2
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many hydrogen atoms are present in a methane molecule?
4
3
2
1
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many total valence electrons are used in the Lewis structure of methane?
4
6
8
10
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where are hydrogen atoms placed in the Lewis structure of methane?
Inside
Below
Above
Outside
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a single line represent in the structural formula of methane?
A triple bond
A pair of bonding electrons
A lone pair
A single electron
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many electrons does hydrogen need for a full outer shell?
1
3
2
4
Similar Resources on Wayground

7 questions
Lewis Dot Structures and Carbon
Interactive video
•
6th - 8th Grade

8 questions
Valence Electrons in Chemistry
Interactive video
•
6th - 8th Grade

8 questions
Valence Electrons and Groups in Periodic Table
Interactive video
•
6th - 8th Grade

8 questions
Valence Electrons in Chemistry
Interactive video
•
6th - 8th Grade

8 questions
Valence Electrons in Chemistry
Interactive video
•
6th - 8th Grade

6 questions
Hydrogen Bonding and Valence Electrons
Interactive video
•
6th - 8th Grade

8 questions
Electron Energy Levels and Valency
Interactive video
•
6th - 9th Grade

7 questions
Valence Electrons and Their Importance
Interactive video
•
6th - 8th Grade
Popular Resources on Wayground

10 questions
SR&R 2025-2026 Practice Quiz
Quiz
•
6th - 8th Grade

30 questions
Review of Grade Level Rules WJH
Quiz
•
6th - 8th Grade

6 questions
PRIDE in the Hallways and Bathrooms
Lesson
•
12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

15 questions
Subtracting Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

10 questions
Physical and chemical properties review
Quiz
•
7th Grade

13 questions
Chemical Vs. Physical Change Level 1
Quiz
•
7th - 8th Grade

20 questions
Pure substances and Mixtures
Quiz
•
8th Grade

20 questions
Metals, Nonmetals, and Metalloids
Quiz
•
6th - 8th Grade

10 questions
Heterogeneous or Homogeneous?
Quiz
•
8th Grade

15 questions
Valence Electron Practice
Quiz
•
8th Grade

30 questions
Atomic Structure and Periodic Table
Quiz
•
8th Grade

41 questions
Atoms, Elements and Compounds
Quiz
•
7th Grade