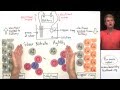
Electroplating Concepts and Processes
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Easy
Mia Campbell
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary reason electroplating is used in making inexpensive jewelry?
To increase the weight of the jewelry
To enhance the durability of the jewelry
To give a precious metal appearance at a lower cost
To make the jewelry more flexible
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the electroplating setup, what is the role of the battery?
To heat the solution
To cool down the metals
To provide a source of electrons
To dissolve the metals
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the term used for the device setup used in electroplating?
Electroplating cell
Voltaic cell
Electrolysis cell
Galvanic cell
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to silver atoms when they lose an electron in the electroplating process?
They become negatively charged ions
They dissolve into the solution as positively charged ions
They remain as neutral atoms
They form a solid layer immediately
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical symbol for silver used in the electroplating process?
Cu
Ag
Au
Fe
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the result of changing a silver atom's charge from neutral to positive?
It becomes a solid metal
It dissolves in the solution
It forms a gas
It becomes magnetic
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do silver ions become neutral atoms on the copper ring?
By gaining a proton
By gaining an electron
By losing a neutron
By losing an electron
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Formative 3BC: Ionic v Covalent Bonds
Quiz
•
9th Grade

10 questions
Exploring Stoichiometry Concepts
Interactive video
•
6th - 10th Grade

20 questions
Mixed Bonding Naming
Quiz
•
9th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Chemical Reactions
Quiz
•
9th Grade

20 questions
Practice: E-Con, Orbital Notation, Noble Gas Notation
Quiz
•
10th Grade

20 questions
Covalent Bonding
Quiz
•
10th Grade