Metathesis Reactions in Organic Chemistry
Interactive Video
•
Chemistry
•
11th - 12th Grade
•
Hard
Aiden Montgomery
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary characteristic of metathesis reactions?
They only involve sigma bonds.
They involve the breaking and forming of bonds in a concerted manner.
They require the presence of a catalyst.
They are irreversible reactions.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In sigma-sigma metathesis, what is essential for the metal involved?
It should be a transition metal.
It must be d0, with no d valence electrons.
It should be in a high oxidation state.
It must have d10 configuration.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a key difference between sigma-sigma and sigma-pi metathesis?
Sigma-sigma involves only pi bonds.
Sigma-pi requires a catalyst.
Sigma-pi results in one molecular entity as the product.
Sigma-sigma is irreversible.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In sigma-pi metathesis, what type of bonds are broken and formed?
Two sigma bonds are broken and two pi bonds are formed.
Two pi bonds are broken and two sigma bonds are formed.
Only pi bonds are involved.
One sigma and one pi bond are broken, forming two new sigma bonds.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
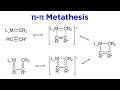
What is unique about pi-pi metathesis compared to other types?
It is not reversible.
It requires a catalyst.
It only involves triple bonds.
The metal does not need to be d0.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which type of metathesis can involve triple bonds?
Sigma-pi metathesis
Pi-pi metathesis
None of the above
Sigma-sigma metathesis
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the example of sigma-sigma metathesis with hafnium, what is the product?
Ethene and a hydride ligand
Ethene and an alkyl ligand
Methane and a hydride ligand
Methane and an alkyl ligand
Create a free account and access millions of resources
Similar Resources on Wayground

8 questions
Pericyclic Reactions 4
Interactive video
•
11th Grade - University

11 questions
Oxidative Addition in Coordination Chemistry
Interactive video
•
11th - 12th Grade

11 questions
Understanding Sigma and Pi Bonds
Interactive video
•
10th - 12th Grade

11 questions
Ligand Interaction and Properties
Interactive video
•
11th - 12th Grade

11 questions
Hybridization and Bonding Concepts
Interactive video
•
10th - 12th Grade

2 questions
Organometallic Reactions Part 6: Metathesis Reactions
Interactive video
•
11th Grade - University

8 questions
Organometallic Reactions Part 2: Oxidative Addition
Interactive video
•
11th Grade - University

7 questions
Hybridization in SO3 Molecule
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
SR&R 2025-2026 Practice Quiz
Quiz
•
6th - 8th Grade

30 questions
Review of Grade Level Rules WJH
Quiz
•
6th - 8th Grade

6 questions
PRIDE in the Hallways and Bathrooms
Lesson
•
12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

15 questions
Subtracting Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

12 questions
significant figures and calculations
Quiz
•
10th - 12th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade

19 questions
U2 Protons Neutrons and Electrons
Quiz
•
11th Grade

20 questions
Lab Safety and Equipment
Quiz
•
9th - 12th Grade

18 questions
Significant Figures Practice
Quiz
•
12th Grade