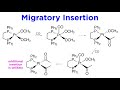
Platinum Complex Reactions and Mechanisms
Interactive Video
•
Chemistry
•
11th - 12th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a necessary condition for migratory insertion to occur between two ligands?
The ligands must be identical.
The ligands must be cis to each other.
The ligands must be trans to each other.
The ligands must be in different coordination spheres.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which type of bond is most commonly involved in migratory insertion?
Sigma bond
Delta bond
Pi bond
Ionic bond
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the context of migratory insertion, what happens when a strong ligand binds to the vacant site?
The ligands become trans to each other.
The insertion becomes irreversible.
The insertion becomes reversible.
The oxidation state of the metal changes.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the result of a hydrometalation reaction?
An alkene becomes an alkyl ligand.
A metal-carbon bond becomes a pi bond.
A ligand dissociates from the metal.
A metal-oxygen bond is formed.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary outcome of carbometalation?
Formation of a nitrile ligand
Formation of a hydride ligand
Formation of a propyl ligand
Formation of a benzyl ligand
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a stable iron complex with 18 electrons, what is unlikely to occur?
Coordination of triphenylphosphine
Ligand dissociation
Change in oxidation state
Migratory insertion
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the preferred electron count for a stable square planar platinum complex?
20 electrons
16 electrons
18 electrons
14 electrons
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Metal-Ligand Interactions and Coordinate Bonds
Interactive video
•
10th - 12th Grade

8 questions
Polynuclear Transition Metal Complexes
Interactive video
•
11th Grade - University

8 questions
Crystal Field Theory
Interactive video
•
11th Grade - University

8 questions
Ligand Field Theory and the Jahn-Teller Effect
Interactive video
•
11th Grade - University

11 questions
Metal Toxicity and Treatment Mechanisms
Interactive video
•
11th - 12th Grade

11 questions
Crystal Field Theory Concepts
Interactive video
•
11th - 12th Grade

11 questions
Crystal Field Theory Quiz
Interactive video
•
10th - 12th Grade

6 questions
The Trans-Effect: Example 2
Interactive video
•
University
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

20 questions
Scientific Notation and Significant Figures
Quiz
•
9th - 12th Grade