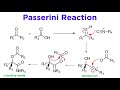
Passerini Reaction and Isonitriles Concepts
Interactive Video
•
Chemistry
•
11th - 12th Grade
•
Hard
Liam Anderson
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the key structural feature of isonitriles that differentiates them from nitriles?
A quadruple bond between nitrogen and carbon
A single bond between nitrogen and carbon
A triple bond between nitrogen and carbon
A double bond between nitrogen and carbon
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why are isonitriles considered kinetically stable despite being less stable than nitriles?
They are more stable thermodynamically
They have a high delta G of activation
They have a low delta G of activation
They are always in equilibrium with nitriles
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the Passerini reaction, what type of product is formed from the combination of three reactants?
Beta-acyloxy amide
Beta-hydroxy amide
Alpha-acyloxy amide
Alpha-hydroxy amide
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the Passerini reaction in library synthesis?
It is used to purify existing compounds
It allows for the synthesis of a single compound
It is primarily used for analytical purposes
It enables the creation of diverse compound libraries
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What characteristic of the Passerini reaction product makes it chiral?
Presence of a stereogenic center
Presence of a prochiral carbonyl group
Use of a chiral catalyst
Formation of a racemic mixture
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the racemic nature of Passerini reaction products not a major concern in library synthesis?
Racemates are less toxic
Racemates are more reactive
Racemates can be easily separated
Racemates are more stable
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the current focus of research in the field of Passerini reactions?
Reducing reaction temperature
Creating enantioselective versions
Improving reaction speed
Developing new reactants
Create a free account and access millions of resources
Similar Resources on Wayground

2 questions
Strecker Amino Acid Synthesis
Interactive video
•
11th Grade - University

2 questions
Ritter Reaction
Interactive video
•
11th Grade - University

2 questions
Biginelli Reaction
Interactive video
•
11th Grade - University

8 questions
Strecker Amino Acid Synthesis
Interactive video
•
11th Grade - University

11 questions
Biginelli Reaction Concepts and Mechanisms
Interactive video
•
11th - 12th Grade

11 questions
Amino Acids and Strecker Synthesis
Interactive video
•
11th - 12th Grade

8 questions
Biginelli Reaction
Interactive video
•
11th Grade - University

4 questions
Strecker Amino Acid Synthesis
Interactive video
•
11th Grade - University
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade

6 questions
Classification of Matter
Lesson
•
9th - 12th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade