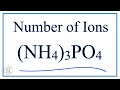
Ammonium Phosphate Ions and Charges
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Aiden Montgomery
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the two ions present in ammonium phosphate?
Ammonium and Nitrate
Ammonium and Phosphate
Sodium and Chloride
Calcium and Carbonate
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of the ammonium ion?
2+
3-
1-
1+
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many ammonium ions are present in ammonium phosphate?
Four
Two
Three
One
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of the phosphate ion?
1+
2-
3-
2+
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many phosphate ions are present in ammonium phosphate?
Four
Three
Two
One
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total positive charge contributed by the ammonium ions in ammonium phosphate?
4+
3+
2+
1+
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of bond is formed between the ions in ammonium phosphate?
Covalent bond
Hydrogen bond
Metallic bond
Ionic bond
Similar Resources on Wayground

6 questions
Ionic Reactions and Equations
Interactive video
•
9th - 10th Grade

6 questions
Calcium and Calcium Phosphate Concepts
Interactive video
•
9th - 10th Grade

6 questions
Ionic Compounds and Phosphate Naming
Interactive video
•
9th - 10th Grade

8 questions
Ammonium Acetate Structure and Properties
Interactive video
•
9th - 10th Grade

6 questions
Calcium Compounds and Ionic Charges
Interactive video
•
9th - 10th Grade

7 questions
Ionic Compounds and Lithium Ions
Interactive video
•
9th - 10th Grade

7 questions
Polyatomic Ions and Their Properties
Interactive video
•
9th - 10th Grade

8 questions
Chemical Formulas and Polyatomic Ions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Electron Configuration & Orbital Notation
Quiz
•
9th - 12th Grade