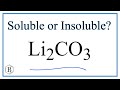
Solubility and Lithium Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which group of elements on the periodic table is generally soluble in water?
Group 1 elements
Group 2 elements
Transition metals
Noble gases
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of a lithium ion?
1-
2-
1+
2+
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to the solubility table, how is lithium carbonate classified?
Insoluble
Slightly soluble
Highly soluble
Not listed
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does 'SS' stand for in the solubility table?
Super Soluble
Slightly Soluble
Solubility Standard
Soluble Solution
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If asked to use solubility rules, how should lithium carbonate be described?
Soluble
Highly insoluble
Slightly soluble
Insoluble
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What should you say if asked about lithium carbonate's solubility using the solubility chart?
Insoluble
Slightly soluble
Soluble
Highly soluble
Similar Resources on Wayground

7 questions
Solubility of Barium Fluoride Concepts
Interactive video
•
9th - 10th Grade

7 questions
Solubility of Metal Oxides
Interactive video
•
9th - 10th Grade

7 questions
Solubility of Copper(II) Fluoride
Interactive video
•
9th - 10th Grade

7 questions
Solubility of Hydroxide Salts
Interactive video
•
9th - 10th Grade

7 questions
Potassium Compounds and Reactions
Interactive video
•
9th - 10th Grade

9 questions
Neutralization Reactions and Properties
Interactive video
•
9th - 10th Grade

6 questions
Solubility and Aqueous Solutions
Interactive video
•
9th - 10th Grade

10 questions
Solubility of Lithium Nitrate and Compounds
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Electron Configuration & Orbital Notation
Quiz
•
9th - 12th Grade