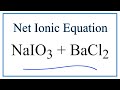
Net Ionic Equations and Spectator Ions
Interactive Video
•
Chemistry
•
10th - 12th Grade
•
Practice Problem
•
Hard
Olivia Brooks
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in writing a balanced net ionic equation for a reaction?
Balance the molecular equation
Determine the solubility of compounds
Write the complete ionic equation
Identify spectator ions
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to know the solubility of compounds in a reaction?
To measure the temperature change
To determine the color of the solution
To identify the precipitate formed
To calculate the reaction rate
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following compounds is insoluble in the reaction between sodium iodate and barium chloride?
Barium chloride
Sodium iodate
Barium iodate
Sodium chloride
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of spectator ions in a chemical reaction?
They increase the reaction rate
They remain unchanged and do not participate
They change the color of the solution
They participate in the reaction
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ions are considered spectator ions in the reaction between sodium iodate and barium chloride?
Barium and iodate ions
Sodium and barium ions
Sodium and chloride ions
Chloride and iodate ions
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of writing a net ionic equation?
To focus on the ions that participate in the reaction
To calculate the reaction yield
To highlight the formation of a precipitate
To show all compounds in the reaction
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is charge conservation ensured in a net ionic equation?
By balancing the number of atoms
By ensuring the total charge is the same on both sides
By adding more ions to the equation
By changing the coefficients of compounds
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

6 questions
Understanding Ionic Equations
Interactive video
•
10th - 12th Grade

11 questions
Understanding the Mole in Chemistry
Interactive video
•
9th - 12th Grade

11 questions
Understanding Periodic Table Trends
Interactive video
•
11th - 12th Grade

9 questions
Understanding Level Curves and Contour Maps
Interactive video
•
10th - 12th Grade

8 questions
Fusion
Interactive video
•
9th - 12th Grade

6 questions
TED-ED: Cell membranes are way more complicated than you think - Nazzy Pakpour
Interactive video
•
KG - University
Popular Resources on Wayground

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
4:3 Model Multiplication of Decimals by Whole Numbers
Quiz
•
5th Grade

10 questions
The Best Christmas Pageant Ever Chapters 1 & 2
Quiz
•
4th Grade

12 questions
Unit 4 Review Day
Quiz
•
3rd Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

15 questions
Solving Equations with Variables on Both Sides Review
Quiz
•
8th Grade
Discover more resources for Chemistry

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Unit 6-Review The Mole
Quiz
•
11th - 12th Grade

20 questions
Unit 3, Quiz #6 Practice - Types of Covalent
Quiz
•
9th - 12th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Exploring Ionic and Covalent Bonding Concepts
Interactive video
•
6th - 10th Grade

148 questions
Fall TEKS Review Chemistry
Quiz
•
9th - 12th Grade

20 questions
Unit 5 - Chemical Reactions Refresh
Quiz
•
9th - 12th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade