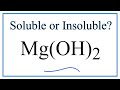
Solubility of Magnesium Hydroxide
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Emma Peterson
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the main question addressed in the video regarding magnesium hydroxide?
Whether it is acidic or basic
Whether it is soluble or insoluble in water
Whether it is a metal or non-metal
Whether it is reactive with air
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to solubility rules, how are most hydroxide salts classified?
Highly reactive
Slightly soluble
Highly soluble
Completely insoluble
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the solubility rules in determining the solubility of compounds?
They determine the melting point
They help identify the state of matter
They provide guidelines on solubility
They predict the color change
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the solubility chart indicate about magnesium hydroxide?
It changes color in water
It reacts violently with water
It is insoluble in water
It is highly soluble in water
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the 'i' symbol on the solubility chart represent for magnesium hydroxide?
It is insoluble
It is highly soluble
It is reactive
It is a gas
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to magnesium hydroxide when placed in water according to the video?
It dissolves completely
It evaporates
It remains suspended
It falls to the bottom
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a practical method mentioned to test the solubility of magnesium hydroxide?
Heating it
Mixing it with oil
Placing it in water
Exposing it to sunlight
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What conclusion does Dr. B reach about the solubility of magnesium hydroxide?
It is highly soluble
It is insoluble or slightly soluble
It is reactive with water
It is a strong acid
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade