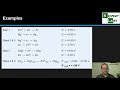
Understanding Cell Voltage and Standard Reduction Potentials
Interactive Video
•
Chemistry
•
10th - 12th Grade
•
Hard

Jennifer Brown
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of a more positive standard reduction potential?
It means the reaction is non-spontaneous.
It shows the reaction will not occur.
It suggests a higher likelihood of reduction.
It indicates a higher likelihood of oxidation.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which formula is used to calculate cell voltage from standard electrode potentials?
E° cell = E° anode - E° cathode
E° cell = E° cathode + E° anode
E° cell = E° cathode - E° anode
E° cell = E° anode + E° cathode
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What must be true for a reaction to be spontaneous in terms of E° cell?
E° cell must be positive.
E° cell must be zero.
E° cell must be negative.
E° cell must be less than zero.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in calculating cell voltage?
Add the half-equations together.
Multiply the half-equations.
Reverse the half-equations.
Write down the two possible reduction half-equations.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why should E° values not be multiplied when balancing equations?
They are only used for oxidation reactions.
They change with temperature.
They are innate properties and do not depend on molarity.
They depend on the number of moles.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the zinc and silver reaction example, which metal acts as the active metal?
Neither
Both
Silver
Zinc
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the calculated cell voltage for the zinc and silver reaction?
1.00 V
0.46 V
0.76 V
1.56 V
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Concentration Cells and Electron Flow
Interactive video
•
10th - 12th Grade

11 questions
Electrochemical Cell Concepts
Interactive video
•
11th - 12th Grade

11 questions
Understanding Galvanic Cells
Interactive video
•
9th - 12th Grade

11 questions
Electroplating Process and Principles
Interactive video
•
9th - 12th Grade

6 questions
J.J. Thomson's Charge-to-Mass Ratio Experiment
Interactive video
•
10th Grade - University

7 questions
Understanding Luminol and Its Applications in Crime Scenes
Interactive video
•
9th - 12th Grade

8 questions
Practice Problem: Cell Potential, Equilibrium Constants, and Free Energy Change
Interactive video
•
11th Grade - University
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade