Molecular Geometry and VSEPR Theory Quiz
Interactive Video
•
Chemistry
•
9th - 12th Grade
•
Hard

Jennifer Brown
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does VSEPR Theory help us understand about molecules?
The color of the molecule
The geometric arrangement of electron groups
The speed of molecular reactions
The weight of the molecule
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
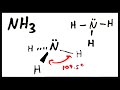
What is the molecular geometry of ammonia (NH3) when lone pairs are considered?
Tetrahedral
Trigonal planar
Linear
Trigonal pyramidal
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the bond angle in NH3 slightly less than the theoretical 109.5°?
Due to the absence of lone pairs
Due to the repulsion caused by lone pairs
Because of the presence of triple bonds
Because of the presence of double bonds
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of CO2?
Tetrahedral
Linear
Trigonal planar
Bent
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does the presence of lone pairs affect the shape of H2O?
It makes the shape linear
It results in a bent shape
It makes the shape trigonal planar
It results in a tetrahedral shape
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the electron geometry of BH3?
Bent
Linear
Trigonal planar
Tetrahedral
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does BH3 have a trigonal planar shape?
Due to the presence of triple bonds
Because it lacks lone pairs
Due to the presence of double bonds
Because it has lone pairs
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Molecular Geometry and Bond Angles
Interactive video
•
9th - 12th Grade

11 questions
Selenium Compounds and Molecular Geometry
Interactive video
•
10th - 12th Grade

11 questions
Molecular Geometry and VSEPR Theory
Interactive video
•
9th - 12th Grade

11 questions
Molecular Geometry and Bond Angles
Interactive video
•
9th - 12th Grade

11 questions
Mastering Molecular Geometry Through VSEPR Theory
Interactive video
•
9th - 12th Grade

11 questions
Predicting Molecular Shapes Using VSEPR Theory
Interactive video
•
9th - 12th Grade

11 questions
Molecular Geometry and Electron Pair Repulsion
Interactive video
•
9th - 12th Grade

11 questions
Molecular Geometry and Bond Angles
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade