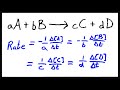
Reaction Rates Quiz
Interactive Video
•
Chemistry
•
10th - 12th Grade
•
Hard

Jennifer Brown
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the initial concentration of NH3 in the given problem?
6 M
2 M
8 M
4 M
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following best describes the rate equation for a reaction?
Rate is the sum of concentrations over time.
Rate is the difference in time over concentration.
Rate is the change in concentration over time.
Rate is the product of concentrations over time.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to balance a chemical equation before calculating reaction rates?
To ensure the reaction is spontaneous.
To find the temperature of the reaction.
To make sure the rate equation is applicable.
To determine the color of the reactants.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the balanced coefficient for NH3 in the given reaction?
4
2
1
3
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the change in concentration of NH3 calculated?
Initial concentration minus final concentration
Final concentration minus initial concentration
Sum of initial and final concentrations
Product of initial and final concentrations
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the time interval given for the reaction?
15 seconds
10 seconds
20 seconds
5 seconds
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the calculated average reaction rate for NH3?
0.01 M/s
0.02 M/s
0.03 M/s
0.04 M/s
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Understanding Rate Constants and Their Units
Interactive video
•
10th - 12th Grade

11 questions
Decomposition of NH3 and Rate Constants
Interactive video
•
11th - 12th Grade

11 questions
Electrolysis and Molar Mass Concepts
Interactive video
•
10th - 12th Grade

11 questions
Chemical Reaction Rates and Orders
Interactive video
•
11th - 12th Grade

6 questions
Learning the Mechanisms : Investigating the Breakdown of Ammonia and Reaction Rates
Interactive video
•
10th Grade - University

11 questions
Chemical Reaction Rates and Constants
Interactive video
•
11th - 12th Grade

11 questions
Concentration Measurement Concepts
Interactive video
•
9th - 12th Grade

11 questions
Buffer Solutions and Equilibrium Concepts
Interactive video
•
11th - 12th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade