Font size
 Worksheets
WorksheetsSCIENCE 6 FIRST PERIODICAL TEST
Total questions: 80
Worksheet time: 3600secs
What are the three states of matter?
A salad is an example of ______.
mixture
element
compound
science
It refers to the space that matter occupies.
volume
mass
weight
density
It is the amount of matter in an object.
volume
mass
weight
density
Which property of matter changes depending on the gravitational pull?
volume
mass
weight
density
Water is a mixture?
yes
no
solute
solvent
What are the properties of matter?
Fog is considered a
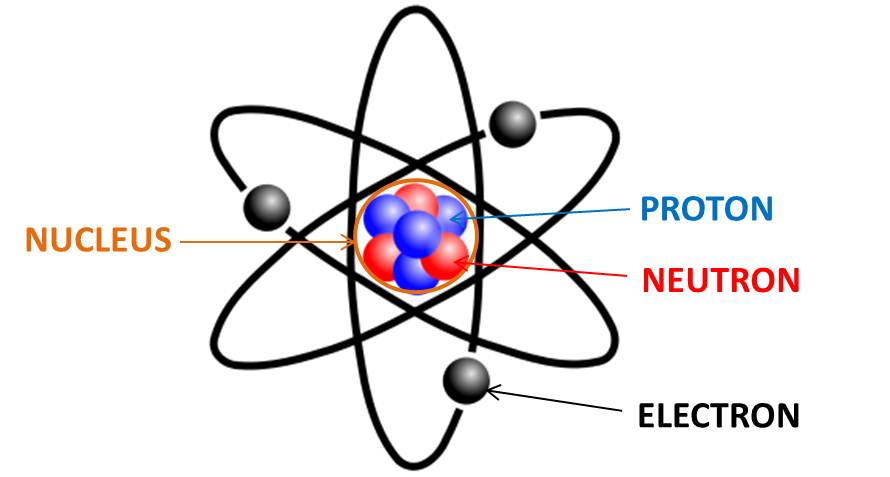
An ______________ is the basic particle from which all elements are made.
Colloids and solutions scatter light
true
false
The scattering of light by colloids is called _____.
the Tyndall effect
conservation
air pollution
suspension
A group of atoms that are held together by chemical forces, it is also the smallest unit of matter that can exist by itself and retain all of a substance's chemical properties.
molecules
atom
compound
elements
Which one is not a colloid
Foam
Gel
Emulsion
Coffee
What is a type of mixture that doesn't separate on its own but still contains undissolved particles
solution
colloid
suspension
solid
A stable mixture of two or more liquids e.g. icecream
emulsion
solution
mixture
insoluble
A substance that can be added to an emulsion to keep the liquids completely mixed
emulsion
emulsifier
emulsification
ammunition
A colloidal solution in which the dispersed phase is a liquid and dispersion medium is a solid is known as ——
In a —— sol there is very little affinity between the dispersed phase and dispersion medium
A substance that stabilises an emulsion is called an ——
The process of bringing a freshly precipitated substance into the colloidal state by adding a suitable reagent is called ——

Perfume spray

Pearl

Toothpaste

Paint

Soap lather

Milk

Mayonnaise
Which of the following statements are true for pure substances? Select any 2 options.
pure substances contain only one kind of particles
pure substances may be compound or mixtures
pure substances have the same composition throughout
Pure substance can be exemplified by all elements other than nickel
It is a type of colloid made out of very small solid particles in a continuous liquid medium. These are quite stable and show the Tyndall effect. One example of this is paint.
Sol
Gel
Aerosol
It is a type of colloid in which the liquid medium has become viscous enough to behave more or less as a solid. An example of this is gummy candy
Gel
Aerosol
Emulsion
A type of Colloid that is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be natural or anthropogenic. An example of this is smoke.
Emulsion
Aerosol
Foam
A type of colloid formed by combining two liquids that normally don't mix. An example of this is mayonaise.
Emulsion
Gel
Mixture
A colloidal system in which the particles are gas bubbles and the medium is a liquid. The term also is applied to material in a lightweight cellular spongy or rigid form. An example of this is Whipped cream.
Foam
Mixture
Matter
It is a homogeneous non-crystalline substance consisting of large molecules or ultramicroscopic particles of one substance dispersed through a second substance. ________________________ include gels, sols, and emulsions; the particles do not settle, and cannot be separated out by ordinary filtering or centrifuging like those in a suspension.
Colloid
Mixture
Matter
An easy way to determine whether a mixture is a colloid or not is through _________________________.
Coffee Effect
Ripple Effect
Tyndall Effect
___________________ refers to substance that spreads particles where the particles are.
Colloid
Dispersing Medium
Rainbow
_________________ means to distribute or spread even over an area.
Bubbles
Bubbles
Disperse
Colloid is with us in our daily life and it is important.
True
False
Also Called Physical manipulation or manual separation
Hand picking
Decantation
Filtration
Use filter like mesh cloth or filter paper to separate solid components from the liquid
Hand picking
Filtration
Decantation
Metals are attracted to magnets. A good way to separate metals with non metals
Use of magnet
Filtration
Hand picking
Used in separating soluble solid from liquid by the use of heat
Condensation
Evaporation
Filtration
This is the oldest method of salt production. It has been used since salt crystals were first noticed in trapped pools of sea water.
Solar Evaporation method
Evaporation method
Condensation Method
One of the approaches taken to isolate the gold from the soil was called _______.
Panning
Winnowing
Shaking
In this method, small particles are aggregated to form colloidal size particles.
Aggregation method
Dispersion method
Methods
A ____________________ reaction is a reaction in which the positive ions and negative ions in two compounds switch partners to form two new compounds.
Double decomposition
Double decomposition
Peptization
______________________is a chemical reaction that involves the gaining of electrons by one of the atoms involved in the reaction between two chemicals.
Reduction
Mechanical Dispersion
Subtraction
____________________ is any chemical reaction that involves the moving of electrons. When iron reacts with oxygen it forms a chemical because it has been oxidized
Oxidation
Reduction
Double decomposition
When iron reacts with oxygen it forms a chemical called _________________ because it has been oxidized (the iron has lost some electrons) and the oxygen has been reduced (the oxygen has gained some electrons).
Dirt
Rust
Trash
An emulsion is a special type of mixture made by combining two liquids that normally don't mix.
True
False
Colloid consists of two parts: colloidal particles and the dispersing medium.
True
False

A WHIPPED CREAM IS AN EXAMPLE OF COLLOID
TRUE
FALSE

sand in water
solution
colloid
suspension
alloy

Sunscreen
solution
colloid
suspension
Alloy
The Tyndall effect is used to distinguish between.
solvents and solutes
liquid and gasses
electrolytes and non electrolytes
solution and colloids
Which one is not a colloid?





What type of colloid is paint
Sol
emulsion
foam
gel

In this beaker there is some sand floating in water, but most of the sand has settled at the bottom. This would be best described as
A solution
A colloid
A suspension
A compound

What type of colloid is milk?
Aerosol
liquid sol
emulsion
liquid foam

What type of colloid is in the picture?
sol
emulsion
foam
gel

What type of colloid is fog?
gel
emulsion
foam
aerosol

What type of colloid is the picture
liquid sol
solid sol
liquid foam
solid foam

BLACK PEPPER, GARLIC AND VINEGAR
SOL
SUS
COL

SOL
SUS
COL
It is an example of homogeneous mixture
orange juice
fruit salad
rice and palay
water and oil
Which has the largest particles?
Solution
Colloids
Suspension
None of the above
Which one is not a colloid?




What is considered the simplest form of matter?
Elements
Compounds
Mixtures
To separate a mixture of salt and water what separation tool/method is used?
Filter
Magnet
Sieve
Evaporation
What tool is used to separate water and sand?
Plastic Bags
Filter
magnet
It is also a colloidal system which mainly consists of charged particles of carbon depressed in air.
Food
Medicine
Smoke Precipitate
Clouds are also colloidal system. In clouds,
water vapors are present in mixture with the dust particles. The water molecules present in cloud have electric charge on them and are of colloidal size.
Water purifier
Sewage disposal
Artificial Rain
It is the process of treating the skins of animals to obtain the leather. Skin of
animals is also a colloidal system in which the
colloidal particles are positively charged.
Leather tanning
Cleansing action of soap
Formation of delta
Extensive deposits of sand and clay
formed at the mouth of any river in sea
at the site where the river falls into sea
are called _______________. As it is very expected, the river water contains colloidal particles of sand and clay.
Smoke screen
Formation of delta
Sewage disposal
These are used to hide something by a
layer of it. In general, it is used to hide the
movement of troops. These are also colloidal
system in which the particles of titanium oxide are
dispersed in air.
Smoke screen
Rubber industry
Sewage disposal
The blue color of sky is due to
scattering of the blue color of sun
light by the colloidal particles of dust
dispersed in the air present in
atmosphere.
True
False
solution is a colloidal system and it removes the oil
and dirt by forming water soluble emulsions.
Cleansing action of soap
Food
Medicine

This involves using a strainer or a big screen to separate the components of solid mixtures.
chromatography
distillation
sieving
physical manipulation

What method of separating mixtures is shown in the picture?
filtration
distillation
physical manipulation
use of magnet

This method is used to separate metals from non metals.
use of magnet
physical manipulation
sieving
chromatography
