
Naming Compounds Lewis Dot Structure
Authored by Cruz Perez
Chemistry
10th Grade
NGSS covered
Used 2+ times

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
What is the correct Lewis Dot Structure for ammonia NH3
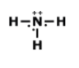
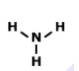
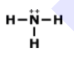
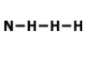
Tags
NGSS.MS-PS1-1
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What is the formula for Lithium Iodide?
Tags
NGSS.MS-PS1-1
3.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
What is the formula for Calcium Chlorate? (refer to your polyatomic ions sheet)
Tags
NGSS.HS-PS1-1
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which applies to KBr?
ionic: potassium bromide
covalent: potassium bromide
ionic: potassium monobromide
covalent: potassium monobromide
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
PCl3
ionic phosphorous trichloride
covalent phosphorous trichloride
Ionic phosphorous chloride
Covalent phosphorous chloride
Tags
NGSS.HS-PS1-1
6.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Why are metals malleable?
They are shiny
The electrons are held tightly within the lattice structure making it strong
The electrons are delocalized and able to move between the atoms
The electrons are shared between two metal ions and this holds the atoms together
Tags
NGSS.HS-PS1-3
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is the correct ionic compound formula for barium nitride?
Ba2N3
Ba2N
BaNO2
Ba3N2
Tags
NGSS.HS-PS1-2
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

15 questions
Chemical and Physical Properties
Quiz
•
8th - 10th Grade

10 questions
displacement of halogen
Quiz
•
9th - 10th Grade

9 questions
Electronic structure and configuration
Quiz
•
6th - 10th Grade

15 questions
ÔN TẬP ĐẦU NĂM HÓA 11
Quiz
•
10th - 11th Grade

15 questions
Concentrations, dilutions, and review
Quiz
•
9th - 12th Grade

10 questions
Periodic Table Quick Review
Quiz
•
9th - 12th Grade

10 questions
Testing for Ions Prelab
Quiz
•
10th Grade

11 questions
Matter and Atomic Structure
Quiz
•
10th - 12th Grade
Popular Resources on Wayground

7 questions
History of Valentine's Day
Interactive video
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

15 questions
Valentine's Day Trivia
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 8 Stoichiometry Review
Quiz
•
10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

19 questions
Stoichiometry, Limiting Reactants, and Percent Yield
Quiz
•
10th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

10 questions
Identifying types of reactions
Quiz
•
9th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade