
Chemistry - Fall Semester Review 2020
Quiz
•
Chemistry
•
10th - 12th Grade
•
Practice Problem
•
Medium
+8
Standards-aligned
April Pence
Used 181+ times
FREE Resource
Enhance your content in a minute
60 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
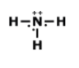
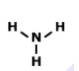
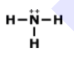
What is the correct Lewis Dot Structure for ammonia NH3
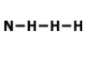
Tags
NGSS.MS-PS1-1
2.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which is the correct molecular structure for carbon dioxide?




Tags
NGSS.MS-PS1-1
3.
MULTIPLE SELECT QUESTION
2 mins • 1 pt
Which of the following elements are an exception to the octet rule and require less than 8 valence electrons?
H
Li
C
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Compounds composed of a ______________ and _____________ like the compound CaCl2 , are ionic compounds.
metal ... metal
nonmetal ... nonmetal
metal ... nonmetal
Tags
NGSS.HS-PS1-2
5.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which of the following describes covalent bonds?
Bonds form because of opposite charges
electrons are shared to fill outer electron shells
Electrons are transferred between atoms
Covalent bonds are magical
Tags
NGSS.HS-PS1-2
6.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Ionic Bonding involves...
Tags
NGSS.HS-PS1-2
7.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Why do atoms form bonds?
to attain a noble gas configuration - STABILITY!
to increase their mass
to increase their atomic number
to attain an alkali metal configuration
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

60 questions
Latsol alkana, alkena, alkuna, isomer, dan reaksi hidrokarbon
Quiz
•
12th Grade

60 questions
Chemistry Final Exam
Quiz
•
10th - 12th Grade

58 questions
Reactivity Series and Displacement Reactions
Quiz
•
9th - 12th Grade

57 questions
Chemical Nomenclature
Quiz
•
10th Grade

62 questions
Szénhidrogének - témazáró
Quiz
•
10th Grade

60 questions
Chemical Reactions and Balancing
Quiz
•
10th Grade

65 questions
AP Chemistry Unit 3
Quiz
•
11th - 12th Grade

57 questions
Yr10 precipitation reactions
Quiz
•
10th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade
Discover more resources for Chemistry

20 questions
Ionic Compound Nomenclature
Quiz
•
9th - 12th Grade

30 questions
ERHS Chem - Chapter 6 Covalent Compounds
Quiz
•
11th Grade

42 questions
Chemistry Final Exam Review
Quiz
•
9th - 12th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

35 questions
Chemistry Semester A Final Review
Quiz
•
11th Grade
