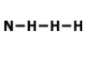U7 Test Review
Quiz
•
Science
•
9th - 12th Grade
•
Medium
Artie Orgeron
Used 5+ times
FREE Resource
Enhance your content in a minute
64 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

The Lewis Dot structure for Oxygen shows ___ valance electrons. Of these, ___ are paired, and ___ are unpaired. Oxygen forms ___ covalent bonds.
6, 2, 4, 2
6, 2, 2, 2
4, 2, 1, 4
4, 1, 2, 4
2.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

Based on the Lewis Dot Structure for the element Oxygen, how many covalent bonds would the O2 molecule have? The Oxygen molecule (O2) make up approximately 19% of our atmosphere.
1
2
3
4
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
A formula with the lowest whole # ratio of elements in a compound is called
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
A chemical formula that shows the actual # and kinds of atoms present in one molecule of a compound is called_________
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Which molecule would have this molecular geometry?
BeCl2
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

What is this shape called?
Trigonal Planar
Tetrahedral
Trigonal Pyramidal
Bent
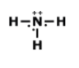
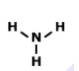
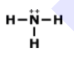
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Who could this molecule be?
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

60 questions
P.S. FINAL EXAM PRACTICE TEST CHEMISTRY FALL '23
Quiz
•
12th Grade

65 questions
Genetics Test Prep
Quiz
•
9th Grade

66 questions
Chemistry of Human Body Review
Quiz
•
9th - 12th Grade

60 questions
Chemical Oceanography Review
Quiz
•
10th Grade

67 questions
B4 AQA Bioenergetics
Quiz
•
KG - 12th Grade

69 questions
AQA Trilogy Chemistry Paper 1 Basics
Quiz
•
9th - 11th Grade

60 questions
The Atom and Periodic Table Test Review
Quiz
•
9th - 12th Grade

65 questions
Physical Science Sem 2 Final Review
Quiz
•
10th Grade
Popular Resources on Wayground

7 questions
History of Valentine's Day
Interactive video
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

15 questions
Valentine's Day Trivia
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Science

25 questions
Naming Ionic and Covalent Compounds
Quiz
•
9th Grade

20 questions
Mendelian Genetics Review
Quiz
•
9th Grade

10 questions
Exploring Weathering, Erosion, and Deposition Processes
Interactive video
•
6th - 10th Grade

10 questions
Exploring Mendelian Genetics and Punnett Squares
Interactive video
•
6th - 10th Grade

10 questions
Exploring Light and Waves Concepts
Interactive video
•
6th - 10th Grade

20 questions
The Cell Cycle and Mitosis
Quiz
•
9th Grade

35 questions
DNA Structure and Replication
Quiz
•
10th Grade

89 questions
Unit 1 (Ch 2 & 3) Test Review - Water/Ocean Currents
Quiz
•
9th - 12th Grade