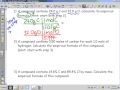
Moles and Stoichiometry: Empirical Formula Challenges
Interactive Video
•
Mia Campbell
•
Chemistry
•
9th - 12th Grade
•
1 plays
•
Medium
05:14
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE
30 sec • 1 pt
What unit is used to convert grams of an element to moles in the dimensional analysis?
2.
MULTIPLE CHOICE
30 sec • 1 pt
What is the gram formula mass (gfm) used for in the conversion process?
3.
MULTIPLE CHOICE
30 sec • 1 pt
What does dimensional analysis help you find in the context of empirical formulas?
4.
MULTIPLE CHOICE
30 sec • 1 pt
What do you need to calculate after finding the moles of each element to determine the empirical formula?
5.
MULTIPLE CHOICE
30 sec • 1 pt
If given moles directly in a problem, what is the next step to find the empirical formula?
6.
MULTIPLE CHOICE
30 sec • 1 pt
How is the empirical formula written after determining the mole ratios?
7.
MULTIPLE CHOICE
30 sec • 1 pt
Why is it important to divide by the lowest mole number when calculating empirical formulas?
8.
MULTIPLE CHOICE
30 sec • 1 pt
What is the final step after calculating the lowest whole number ratio in empirical formula derivation?
9.
MULTIPLE CHOICE
30 sec • 1 pt
What is the first step in deriving an empirical formula if you start with percent composition?
10.
MULTIPLE CHOICE
30 sec • 1 pt
What is the purpose of assuming a 100 gram sample when starting with percent composition?
Explore all questions with a free account
Popular Resources on Quizizz

5 questions
Brain Teasers
•
KG - University

37 questions
Math STAAR Review
•
4th Grade

12 questions
Earth Day
•
4th Grade

25 questions
STAAR REVIEW - SCIENCE
•
5th Grade

20 questions
Science STAAR Review! 23-24
•
5th Grade

20 questions
Reading Comprehension
•
5th Grade

20 questions
Types of Credit
•
9th - 12th Grade

23 questions
Math STAAR Review
•
4th Grade
Discover more resources for Chemistry

10 questions
Energy & Energy Transformations
•
9th - 12th Grade

20 questions
Chemical and Physical changes
•
10th - 12th Grade

62 questions
K Intro to REDOX pg1-4
•
10th Grade

15 questions
Endothermic and Exothermic Reactions
•
9th Grade

10 questions
Gas laws (Boyle's, Charles', Lussac)
•
9th Grade

52 questions
Unit 8: Gas Laws Review
•
10th Grade

23 questions
Boyle's, Charles', Gay-Lussac Laws
•
10th Grade

15 questions
Molarity & Dilutions
•
10th Grade