
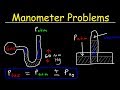
Understanding Manometers and Barometers
Interactive Video
•
Physics, Science
•
9th - 12th Grade
•
Practice Problem
•
Hard
Mia Campbell
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the gas pressure in a manometer if the atmospheric pressure is 760 mmHg and the mercury column is 60 mm higher on the atmospheric side?
760 mmHg
820 mmHg
700 mmHg
680 mmHg
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How can you determine if the gas pressure is greater than the atmospheric pressure in a manometer?
By measuring the temperature
By checking the color of the mercury
By comparing the height of the mercury column
By observing the speed of gas particles
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If the mercury column is 50 mm lower on the atmospheric side, what is the gas pressure?
760 mmHg
700 mmHg
710 mmHg
810 mmHg
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the mercury column when an empty test tube is inverted in a container with mercury?
The mercury column disappears
The mercury column remains unchanged
The mercury column falls
The mercury column rises
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does the mercury column not change when an empty test tube is inverted?
Because the atmospheric pressure is too low
Because the mercury is too heavy
Because there are gas particles inside the test tube
Because the test tube is sealed
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is created inside the test tube when it is filled with mercury and inverted?
A gas-filled space
A vacuum
A high-pressure zone
A liquid-filled space
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary function of a barometer?
To measure atmospheric pressure
To measure gas pressure
To measure temperature
To measure humidity
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Michael Faraday and Electromagnetism
Interactive video
•
9th - 12th Grade

11 questions
Understanding Surface Tension
Interactive video
•
9th - 12th Grade

11 questions
Entropy and Spontaneous Processes
Interactive video
•
10th - 12th Grade

11 questions
Understanding Chemical Solutions and Osmosis
Interactive video
•
10th - 12th Grade

11 questions
Mathematical Modeling in Structural Geology
Interactive video
•
10th Grade - University

6 questions
China's Transition to Renewable Energy
Interactive video
•
9th - 12th Grade

6 questions
Observations and Data: Displaying Data
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

10 questions
Probability Practice
Quiz
•
4th Grade

15 questions
Probability on Number LIne
Quiz
•
4th Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

6 questions
Appropriate Chromebook Usage
Lesson
•
7th Grade

10 questions
Greek Bases tele and phon
Quiz
•
6th - 8th Grade
Discover more resources for Physics

10 questions
Exit Check 3.3 - Universal Gravitation
Quiz
•
9th Grade

10 questions
Exit Check 3.4 - Moon's Orbit
Quiz
•
9th Grade

10 questions
Exit Check 3.5 - Earth's Orbit
Quiz
•
9th Grade

22 questions
Waves
Quiz
•
KG - University

21 questions
EM Spectrum
Quiz
•
6th - 9th Grade

20 questions
Position vs. Time Graphs
Quiz
•
9th Grade

10 questions
Exploring the Properties of Waves
Interactive video
•
9th - 12th Grade

14 questions
Bill Nye Waves
Interactive video
•
9th - 12th Grade